Quality assessment
From MedITEX - Wiki
| Line 3: | Line 3: | ||
<h2>Quality assessment settings</h2> | <h2>Quality assessment settings</h2> | ||
<p>Before using <strong>Quality assessment</strong>, check the basic parameters in the settings of MedITEX IVF and adjust if needed.</p> | <p>Before using <strong>Quality assessment</strong>, check the basic parameters in the settings of MedITEX IVF and adjust if needed.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 21: | Line 21: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p>As number of differentiating cells for <strong>motility </strong>and <strong>morphology </strong>by default <strong>200 sperm</strong> are set. This number is the minimum value that is to be counted according to the directive per sperm count.</p> | <p>As number of differentiating cells for <strong>motility </strong>and <strong>morphology </strong>by default <strong>200 sperm</strong> are set. This number is the minimum value that is to be counted according to the directive per sperm count.</p> | ||
<p>Also the specification of the <strong>workplace</strong>, the <strong>examination </strong>and the <strong>method </strong>should be entered here. The data will be <strong>automatically </strong>transferred to your Quality assessment entry screen.</p> | <p>Also the specification of the <strong>workplace</strong>, the <strong>examination </strong>and the <strong>method </strong>should be entered here. The data will be <strong>automatically </strong>transferred to your Quality assessment entry screen.</p> | ||
| Line 27: | Line 28: | ||
<h2>Using Quality assessment</h2> | <h2>Using Quality assessment</h2> | ||
<p>The Quality assessment functionality, you use at the menu item <strong>"Laboratory diagnosis"</strong> of the male patients in which you create a new semen analysis by using the button <strong>"New sample"</strong>.</p> | <p>The Quality assessment functionality, you use at the menu item <strong>"Laboratory diagnosis"</strong> of the male patients in which you create a new semen analysis by using the button <strong>"New sample"</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/QM3.png" alt="" width="279" height="329" /></td> | <td><img src="/images/QM3.png" alt="" width="279" height="329" /></td> | ||
| − | <td><img src="/images/QM4.png" alt=" | + | <td><img src="/images/QM4.png" alt="" /></td> |
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p><strong>Examinations </strong>can be carried out either <strong>manually </strong>or <strong>automatically </strong>(CASA = Sperm class analyzer).<br /><br />The <strong>examination </strong>of the spermatozoa in a counting chamber is separated according to</p> | <p><strong>Examinations </strong>can be carried out either <strong>manually </strong>or <strong>automatically </strong>(CASA = Sperm class analyzer).<br /><br />The <strong>examination </strong>of the spermatozoa in a counting chamber is separated according to</p> | ||
<ol> | <ol> | ||
| Line 42: | Line 44: | ||
</ol> | </ol> | ||
<p>performed in <strong>duplicate determinations</strong>, <strong>evaluated </strong>and <strong>documented</strong>.</p> | <p>performed in <strong>duplicate determinations</strong>, <strong>evaluated </strong>and <strong>documented</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 50: | Line 52: | ||
</table> | </table> | ||
<p style="text-align: center;">This form is<strong> no direct data entry</strong> in fields<br /><br />Concentration<br />Motility and<br />Morphology<br /><br />possible, because a <strong>duplicate determination</strong> is required.</p> | <p style="text-align: center;">This form is<strong> no direct data entry</strong> in fields<br /><br />Concentration<br />Motility and<br />Morphology<br /><br />possible, because a <strong>duplicate determination</strong> is required.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 57: | Line 59: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p>The <strong>examintaions </strong>are independent according to the <strong>quantity mass</strong> and can be <strong>evaluated separately</strong>.</p> | <p>The <strong>examintaions </strong>are independent according to the <strong>quantity mass</strong> and can be <strong>evaluated separately</strong>.</p> | ||
<p> </p> | <p> </p> | ||
| Line 66: | Line 69: | ||
<li>Subsequently, the two halves of counting chambers are evaluated separately. <strong>Each counting chamber is one of the duplicate determinations!</strong></li> | <li>Subsequently, the two halves of counting chambers are evaluated separately. <strong>Each counting chamber is one of the duplicate determinations!</strong></li> | ||
</ol> | </ol> | ||
| − | <table border="0" width="587" height="68"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="587" height="68"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 84: | Line 87: | ||
<h2>Execution - investigation motility & morphology</h2> | <h2>Execution - investigation motility & morphology</h2> | ||
<p>In the <strong>duplicate determinations</strong> at least <strong>2x200 sperm</strong> have to be evaluated. If the number of sperm less than 200/visulal field <strong>after enrichment</strong>, the requirement is not relevant and a <strong>smaller number</strong> has to be evaluated.</p> | <p>In the <strong>duplicate determinations</strong> at least <strong>2x200 sperm</strong> have to be evaluated. If the number of sperm less than 200/visulal field <strong>after enrichment</strong>, the requirement is not relevant and a <strong>smaller number</strong> has to be evaluated.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 91: | Line 94: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<h3>Investigation motility</h3> | <h3>Investigation motility</h3> | ||
<p>The <strong>examination </strong>of the sperm is to perform with <strong>motility </strong>(movement in 3 stages:<strong> progressive movement</strong>, <strong>locally movable</strong> and <strong>immotil </strong>in percent) in <strong>duplicate determinations</strong>.</p> | <p>The <strong>examination </strong>of the sperm is to perform with <strong>motility </strong>(movement in 3 stages:<strong> progressive movement</strong>, <strong>locally movable</strong> and <strong>immotil </strong>in percent) in <strong>duplicate determinations</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 100: | Line 104: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p>In Germany entering the values of WHO A and WHO B must be separated because DIR interface!</p> | <p>In Germany entering the values of WHO A and WHO B must be separated because DIR interface!</p> | ||
| − | + | <table style="margin-left: auto; margin-right: auto;" border="0"> | |
| − | <table border="0"> | + | |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 109: | Line 113: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p>There is a "<strong>WHO sperm calculator</strong>", that converts the numbers in percent. New is the "<strong>Motility counter</strong>" that counts the respective sperm about the keyboard, or via an external number pad touch screen monitor. (freely configurable).</p> | <p>There is a "<strong>WHO sperm calculator</strong>", that converts the numbers in percent. New is the "<strong>Motility counter</strong>" that counts the respective sperm about the keyboard, or via an external number pad touch screen monitor. (freely configurable).</p> | ||
<p><span id="result_box" lang="en"><span class="hps">To</span> <span class="hps">learn more about</span> <span class="hps">the</span> <span class="hps atn">"Motility counter</span><span>"</span><span>, click <a href="/index.php?title=Motility_counter">here</a></span><span>.</span></span></p> | <p><span id="result_box" lang="en"><span class="hps">To</span> <span class="hps">learn more about</span> <span class="hps">the</span> <span class="hps atn">"Motility counter</span><span>"</span><span>, click <a href="/index.php?title=Motility_counter">here</a></span><span>.</span></span></p> | ||
| + | <p><span lang="en"><span><br /></span></span></p> | ||
<h3>Investigation morphology</h3> | <h3>Investigation morphology</h3> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 128: | Line 134: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p>The <strong>investigation </strong>of sperm <strong>morphology </strong>is to be carried out (normal in percent) in a <strong>duplicate determination</strong>.</p> | <p>The <strong>investigation </strong>of sperm <strong>morphology </strong>is to be carried out (normal in percent) in a <strong>duplicate determination</strong>.</p> | ||
<p> </p> | <p> </p> | ||
<h2>Documentation</h2> | <h2>Documentation</h2> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 155: | Line 162: | ||
<h2>Result</h2> | <h2>Result</h2> | ||
<p>For each duplicate determination the <strong>evaluation of results</strong> have to be made <strong>immediately after</strong> the completion of investigations.<br /><br />Please open the second tab <strong>"Result"</strong>, which is located right next to the tab<strong> "Sperm counts"</strong>.</p> | <p>For each duplicate determination the <strong>evaluation of results</strong> have to be made <strong>immediately after</strong> the completion of investigations.<br /><br />Please open the second tab <strong>"Result"</strong>, which is located right next to the tab<strong> "Sperm counts"</strong>.</p> | ||
| + | <p> </p> | ||
<h3>Result of the sperm concentration</h3> | <h3>Result of the sperm concentration</h3> | ||
<p>Parameters: number of sperm in the two count chambers of the duplicate determination.</p> | <p>Parameters: number of sperm in the two count chambers of the duplicate determination.</p> | ||
| − | + | <table style="margin-left: auto; margin-right: auto;" border="0"> | |
| − | <table border="0"> | + | |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 176: | Line 183: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/Q15.png" alt=" | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/Q15.png" alt="" /></td> |
<td>If the difference is greater than the formula value,<br />the release can not be effected. The count have to carried out again.</td> | <td>If the difference is greater than the formula value,<br />the release can not be effected. The count have to carried out again.</td> | ||
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<h3>Result of the sperm motility</h3> | <h3>Result of the sperm motility</h3> | ||
<p><strong>Each type of sperm (WHO AB, C WHO, WHO D)</strong> has to be evaluated.<br /><strong>Parameters: percentage</strong> P1 and P2 of one type, first and second counting.</p> | <p><strong>Each type of sperm (WHO AB, C WHO, WHO D)</strong> has to be evaluated.<br /><strong>Parameters: percentage</strong> P1 and P2 of one type, first and second counting.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 206: | Line 214: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<h3>Result of the motility</h3> | <h3>Result of the motility</h3> | ||
<div id="gt-res-content" class="almost_half_cell"> | <div id="gt-res-content" class="almost_half_cell"> | ||
<div style="zoom: 1;" dir="ltr"><span id="result_box" lang="en"><strong><span class="hps">Parameters:</span> <span class="hps">percentage</span> </strong><span class="hps">P1 and P2</span> <span class="hps">of the normal</span> <span class="hps">sperm.</span></span></div> | <div style="zoom: 1;" dir="ltr"><span id="result_box" lang="en"><strong><span class="hps">Parameters:</span> <span class="hps">percentage</span> </strong><span class="hps">P1 and P2</span> <span class="hps">of the normal</span> <span class="hps">sperm.</span></span></div> | ||
</div> | </div> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 237: | Line 246: | ||
<p>Exist more than <strong>50 shared value pairs</strong>, after a <strong>control period</strong> (= 1 calendar month), or - if this number is not reached - <strong>when 50 pairs be achieved</strong>, a <strong>long-term validation </strong>by calculating and evaluating the mean (including standard deviation) have to be made of the d<strong>uplicate determinations</strong>.</p> | <p>Exist more than <strong>50 shared value pairs</strong>, after a <strong>control period</strong> (= 1 calendar month), or - if this number is not reached - <strong>when 50 pairs be achieved</strong>, a <strong>long-term validation </strong>by calculating and evaluating the mean (including standard deviation) have to be made of the d<strong>uplicate determinations</strong>.</p> | ||
<p><span id="result_box" lang="en"><span class="hps">To do</span> <span class="hps">this, click</span> <span class="hps"><strong>Reports -></strong></span><strong> <span class="hps">Semen analysis quality assessment<br /></span></strong></span></p> | <p><span id="result_box" lang="en"><span class="hps">To do</span> <span class="hps">this, click</span> <span class="hps"><strong>Reports -></strong></span><strong> <span class="hps">Semen analysis quality assessment<br /></span></strong></span></p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/QM18.png" alt=" | + | <td><img src="/images/QM18.png" alt="" /></td> |
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<h3>Long-term validation preparation</h3> | <h3>Long-term validation preparation</h3> | ||
<p>All results of the internal quality assurance are documented separately by <strong>paramenter </strong>and <strong>workplace</strong>.</p> | <p>All results of the internal quality assurance are documented separately by <strong>paramenter </strong>and <strong>workplace</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 269: | Line 279: | ||
<p> </p> | <p> </p> | ||
<p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | <p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 286: | Line 296: | ||
<p> </p> | <p> </p> | ||
<p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | <p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 297: | Line 307: | ||
</table> | </table> | ||
<p> </p> | <p> </p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 311: | Line 321: | ||
<p> </p> | <p> </p> | ||
<p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | <p>All <strong>results </strong>of internal quality assessment have to be <strong>documented separately</strong> by <strong>parameter </strong>and <strong>workplace</strong>.</p> | ||
| − | <table border="0" width="662" height="74"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="662" height="74"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 323: | Line 333: | ||
<p> </p> | <p> </p> | ||
<p>Is the <strong>mean value of the difference greater than the formula value</strong>, may no results be issued, <strong>until the cause is cleared and removed</strong>. The process must be <strong>documented</strong>. The fields <strong>cause, corrective action, eliminated</strong> and <strong>person (correction)</strong> need to be completed. Is this not the case, <strong>"information messages"</strong> remember on it.</p> | <p>Is the <strong>mean value of the difference greater than the formula value</strong>, may no results be issued, <strong>until the cause is cleared and removed</strong>. The process must be <strong>documented</strong>. The fields <strong>cause, corrective action, eliminated</strong> and <strong>person (correction)</strong> need to be completed. Is this not the case, <strong>"information messages"</strong> remember on it.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 333: | Line 343: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | |||
<table style="float: right;" border="0"> | <table style="float: right;" border="0"> | ||
<tbody> | <tbody> | ||
Revision as of 13:18, 9 January 2013
This link leads you to the German version "<a href="/index.php?title=RiliB%C3%84K%20zu%20Ejakulatuntersuchungen">RiliBÄK</a>".
Contents |
Quality assessment settings
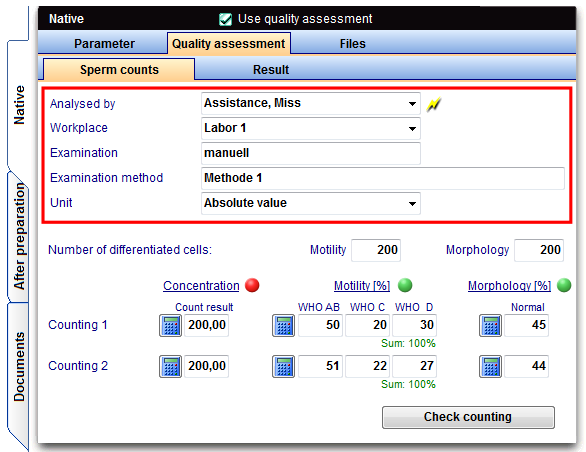
Before using Quality assessment, check the basic parameters in the settings of MedITEX IVF and adjust if needed.
| <img src="/images/QM1.png" alt="" width="508" height="119" /> |
- You access settings via the menu System -> Configuration / administration -> Settings on the top of the window.
- In the settings you go to System -> Functions -> Semen analysis to Quality assessment-default settings and check "Activate / deactivate Qualitiy assessment".
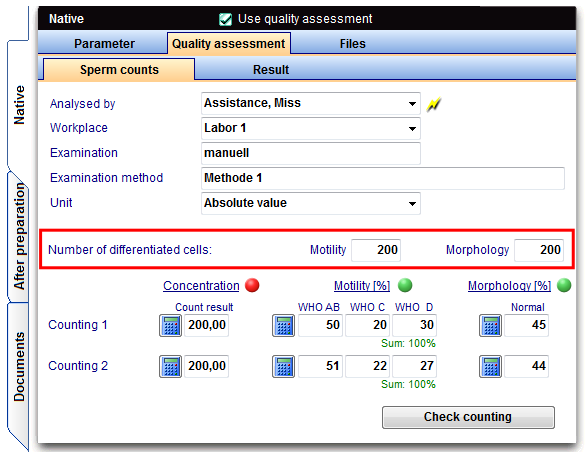
As number of differentiating cells for motility and morphology by default 200 sperm are set. This number is the minimum value that is to be counted according to the directive per sperm count.
Also the specification of the workplace, the examination and the method should be entered here. The data will be automatically transferred to your Quality assessment entry screen.
The dilution or enrichment of the ejaculate and / or the number of the counting fields must be selected in accordance with an orienting preliminary examination. If the sperm concentration is less than 1-2 sperm per high power field (objective magnification 40-fold), the sample should be enriched. Finds then less than 200 sperm per counting grid of the counting chamber, eliminates the requirement of at least 2 x 200 to be counted spermatozoa.
These settings will be loaded on every Quality assessment calculation as default values and can be edited in addition, depending on the special case.
Using Quality assessment
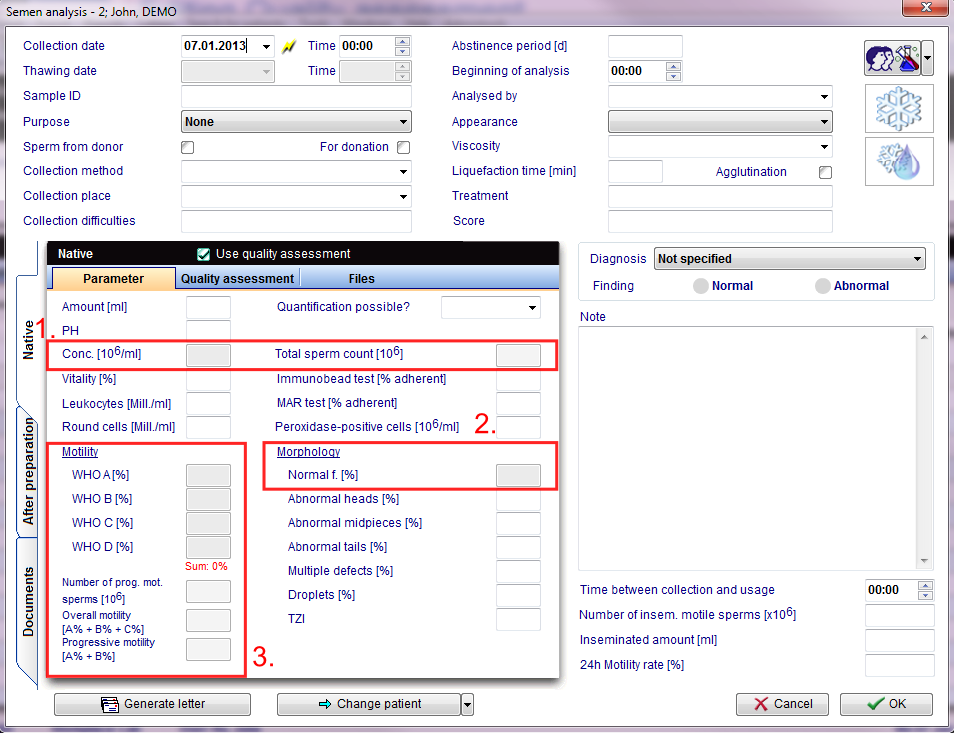
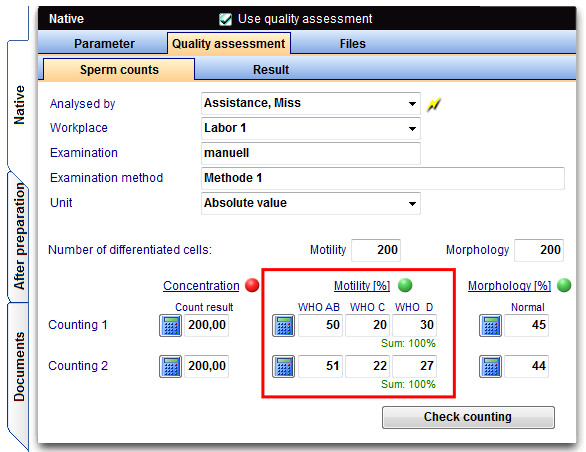
The Quality assessment functionality, you use at the menu item "Laboratory diagnosis" of the male patients in which you create a new semen analysis by using the button "New sample".
| <img src="/images/QM3.png" alt="" width="279" height="329" /> | <img src="/images/QM4.png" alt="" /> |
Examinations can be carried out either manually or automatically (CASA = Sperm class analyzer).
The examination of the spermatozoa in a counting chamber is separated according to
- Concentration (number),
- Morphology (normal or abnormal in percent)
- Motility (movement in 3 stages: progressive movement, locally movable immotil in percent)
performed in duplicate determinations, evaluated and documented.
This form is no direct data entry in fields
Concentration
Motility and
Morphology
possible, because a duplicate determination is required.
The examintaions are independent according to the quantity mass and can be evaluated separately.
Execution - investigation concentration
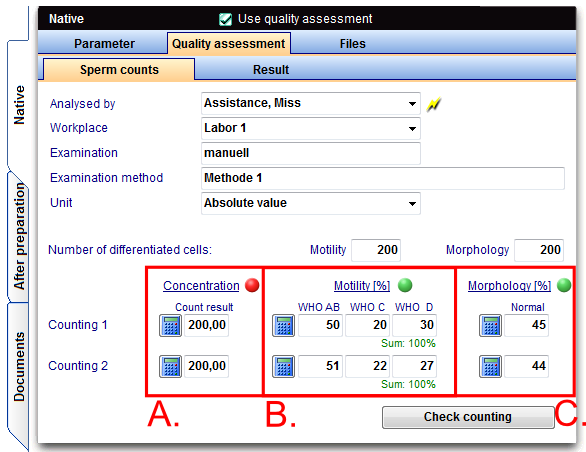
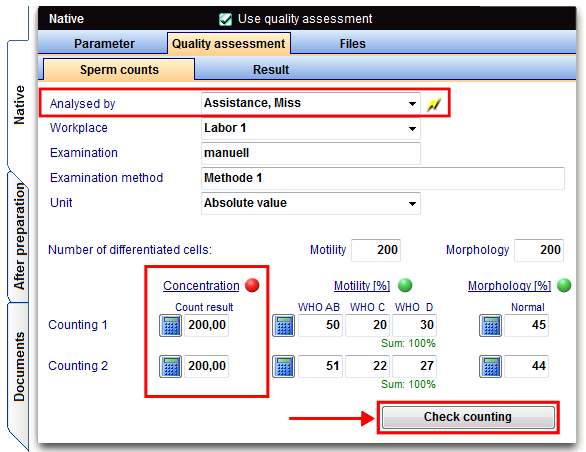
To add a count with duplicate determination go to the tab Quality assessment-> Sperm counts.
The duplicate determination of the concentration is carried out as follows:
- A counting chamber is filled with the sample.
- Subsequently, the two halves of counting chambers are evaluated separately. Each counting chamber is one of the duplicate determinations!
|
Execution - investigation motility & morphology
In the duplicate determinations at least 2x200 sperm have to be evaluated. If the number of sperm less than 200/visulal field after enrichment, the requirement is not relevant and a smaller number has to be evaluated.
Investigation motility
The examination of the sperm is to perform with motility (movement in 3 stages: progressive movement, locally movable and immotil in percent) in duplicate determinations.
In Germany entering the values of WHO A and WHO B must be separated because DIR interface!
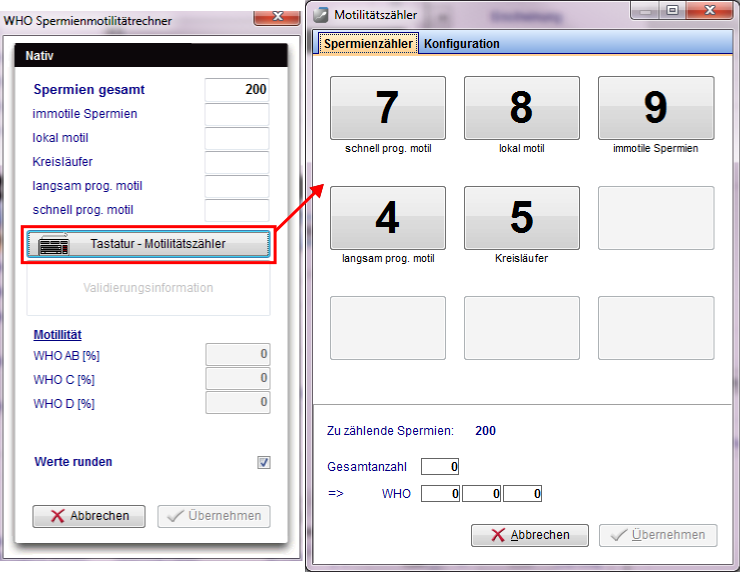
There is a "WHO sperm calculator", that converts the numbers in percent. New is the "Motility counter" that counts the respective sperm about the keyboard, or via an external number pad touch screen monitor. (freely configurable).
To learn more about the "Motility counter", click <a href="/index.php?title=Motility_counter">here</a>.
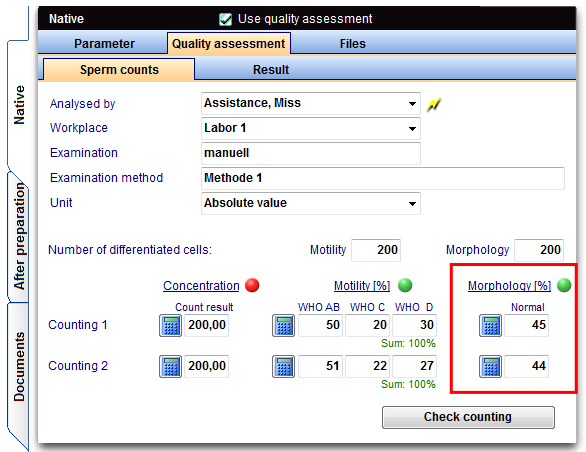
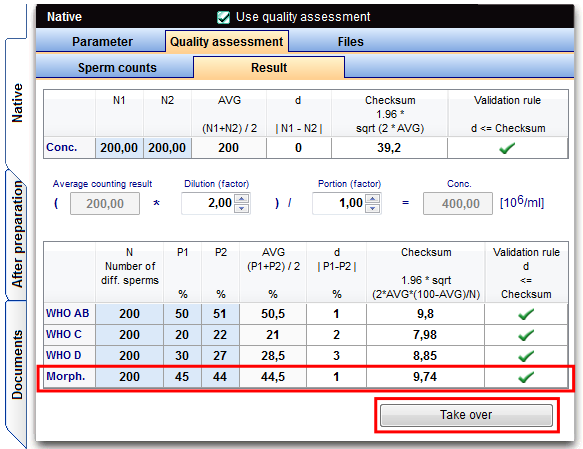
Investigation morphology
|
Morphology (normal forms)
|
The investigation of sperm morphology is to be carried out (normal in percent) in a duplicate determination.
Documentation
|
The content of the documentation provided (excerpt):
|
Result
For each duplicate determination the evaluation of results have to be made immediately after the completion of investigations.
Please open the second tab "Result", which is located right next to the tab "Sperm counts".
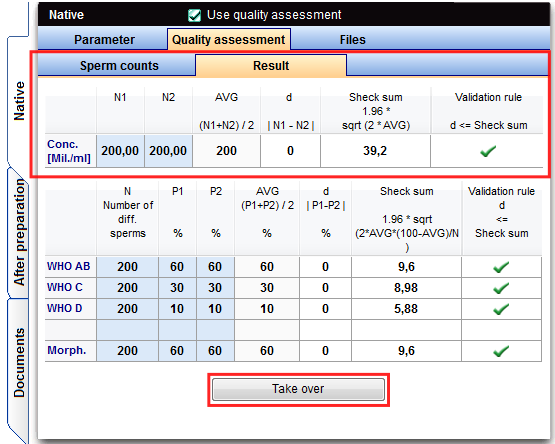
Result of the sperm concentration
Parameters: number of sperm in the two count chambers of the duplicate determination.
|
|
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/Q15.png" alt="" /> | If the difference is greater than the formula value, the release can not be effected. The count have to carried out again. |
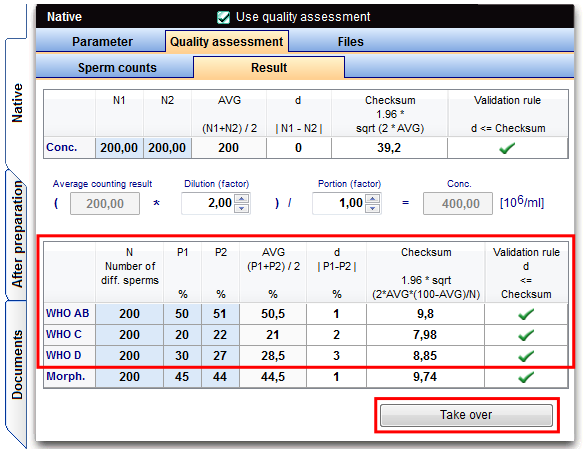
Result of the sperm motility
Each type of sperm (WHO AB, C WHO, WHO D) has to be evaluated.
Parameters: percentage P1 and P2 of one type, first and second counting.
Assessment: d ≤ 1,96 * sqrt( 2 * MW * (100-MW) : N ) |
|
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/Q15.png" alt="" width="119" height="88" /> | If the difference is greater than the formula value, the release can not be effected. The count have to carried out again. |
Result of the motility
Assessment: d ≤ 1,96 * sqrt( 2 * MW * (100-MW) : N ) |
|
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/Q15.png" alt="" width="119" height="88" /> | If the difference is greater than the formula value, the release can not be effected. The count have to carried out again. |
Semen analysis quality assessment
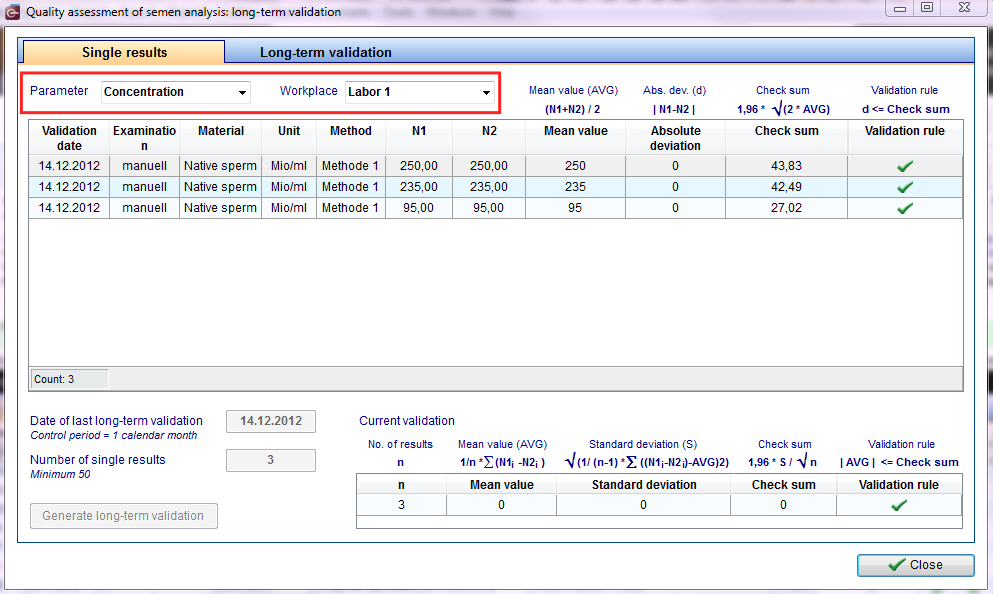
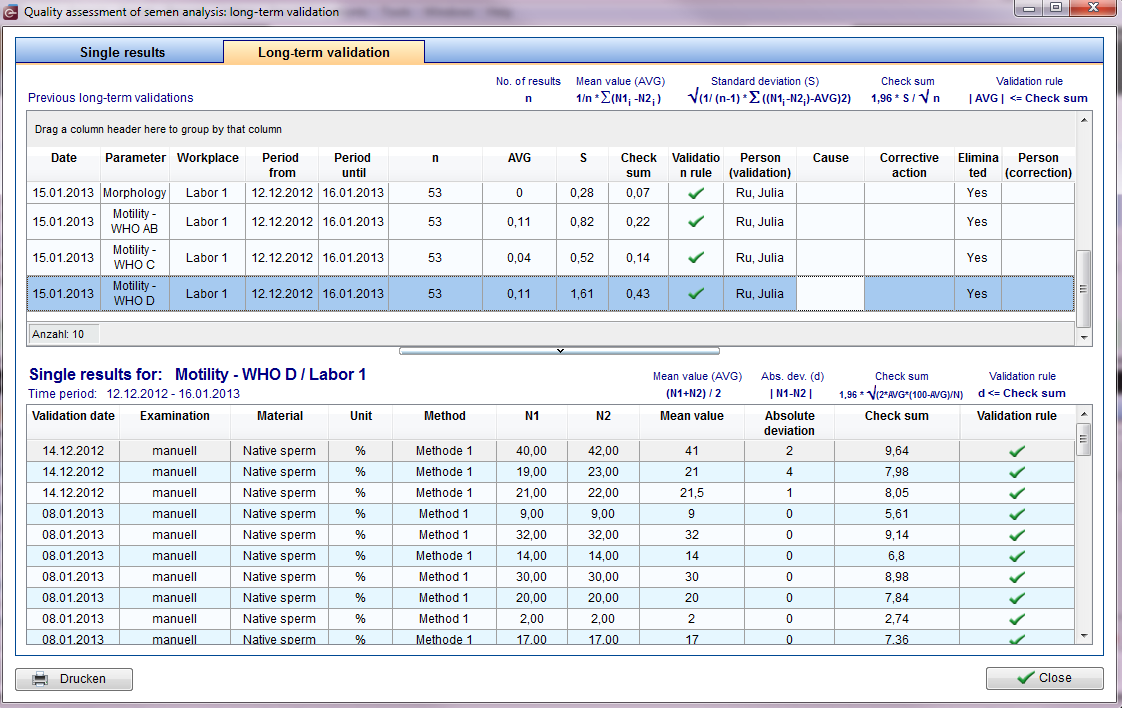
Exist more than 50 shared value pairs, after a control period (= 1 calendar month), or - if this number is not reached - when 50 pairs be achieved, a long-term validation by calculating and evaluating the mean (including standard deviation) have to be made of the duplicate determinations.
To do this, click Reports -> Semen analysis quality assessment
| <img src="/images/QM18.png" alt="" /> |
Long-term validation preparation
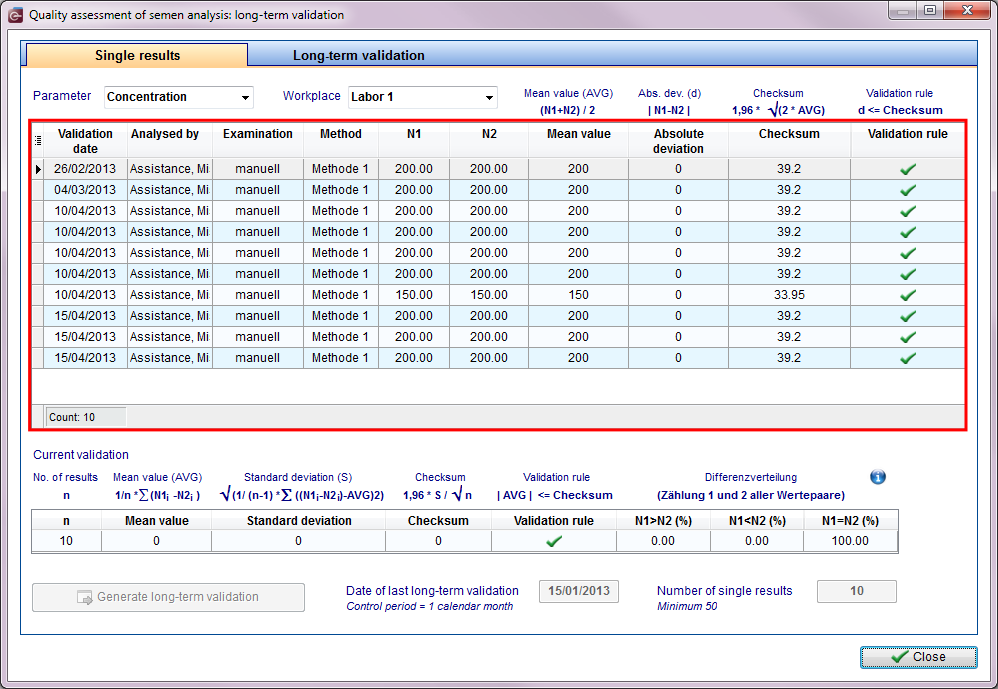
All results of the internal quality assurance are documented separately by paramenter and workplace.
|
First Step: Select parameter and workplace Parameters:
It can be displayed only a single quantity! |
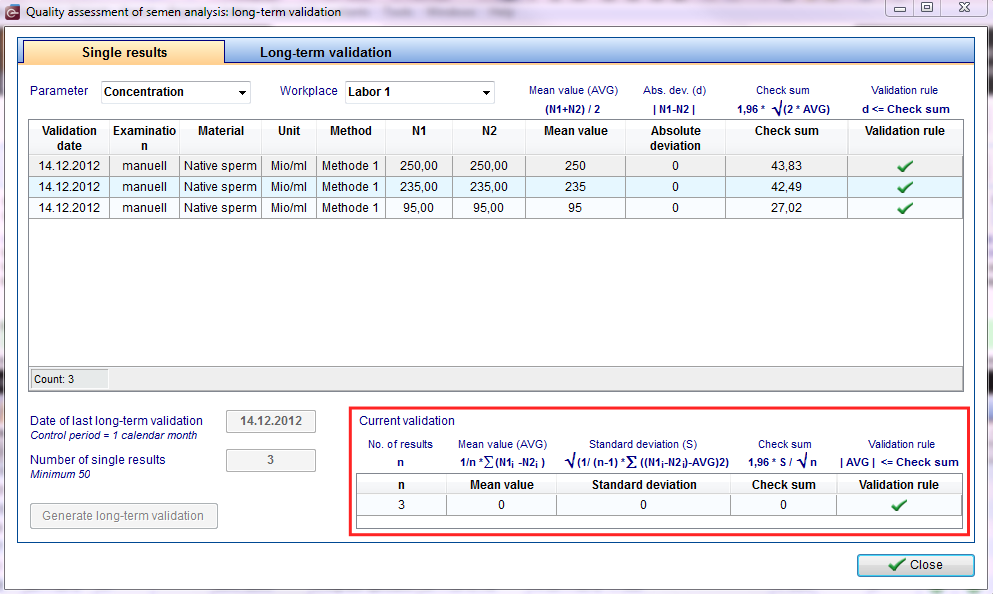
All results of internal quality assessment have to be documented separately by parameter and workplace.
Second Step: evaluation of all duplicate determinations
|
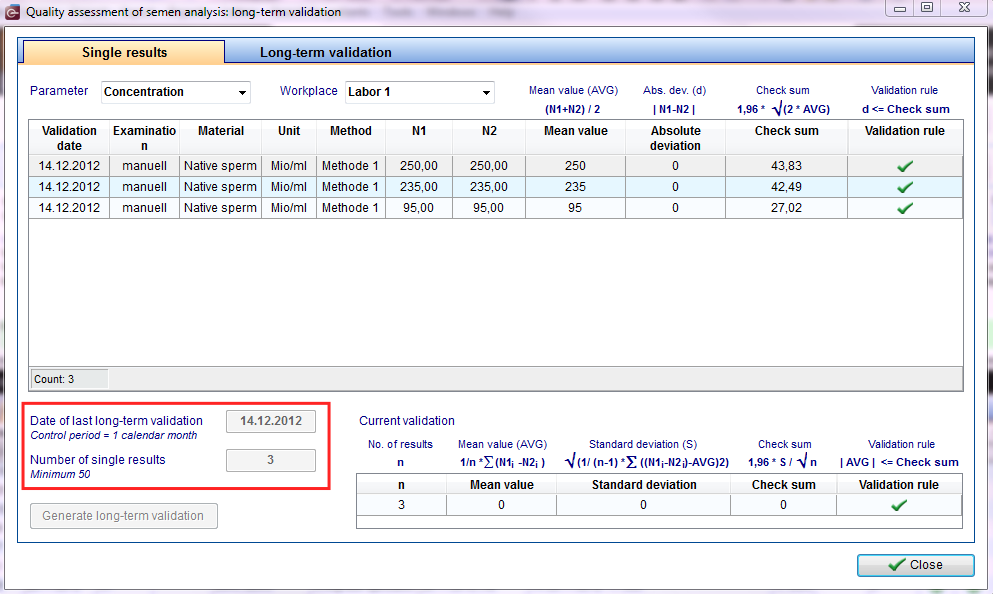
All results of internal quality assessment have to be documented separately by parameter and workplace.
| Third Step: flowing assessment/ current assessment n = number of pairs of values (immediately reviews) d = difference of the value pairs MWd = mean deviation = 1: n * Sum (d) Sd = standard deviation of the difference = sqrt (1: (n-1) * Sum ([d MWd] 2)) Assessment: MWd ≤ 1.96 * sd: sqrt (n) |
|
Fourth Step: control period / 50 value pairs |
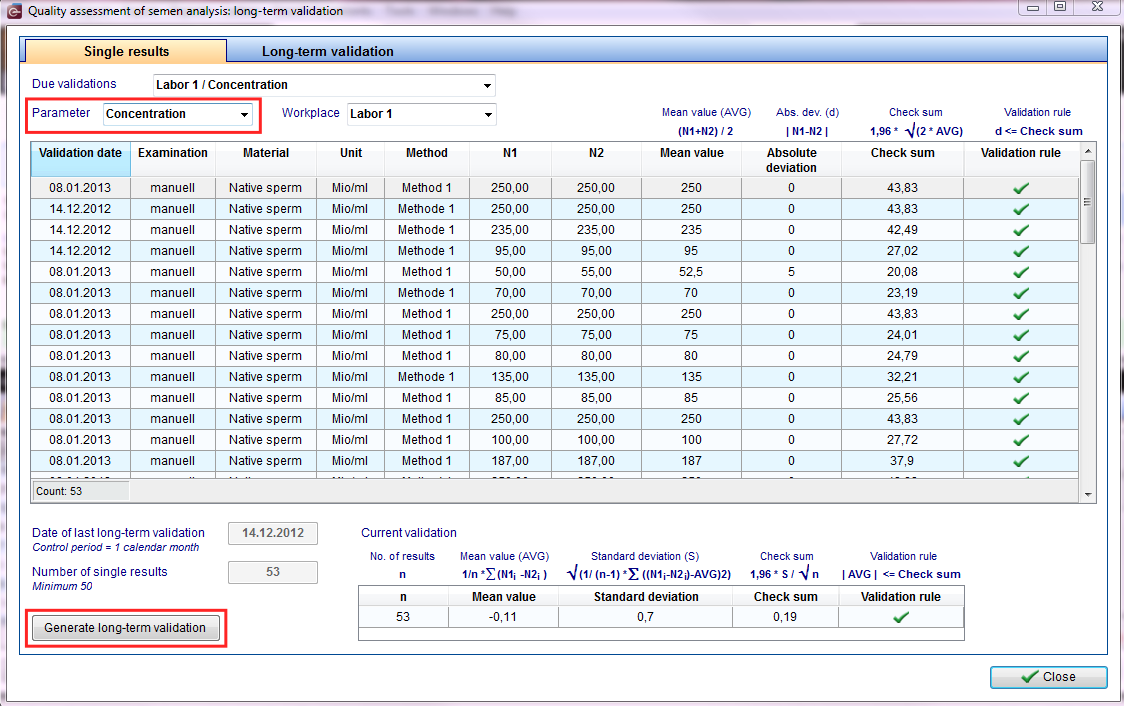
All results of internal quality assessment have to be documented separately by parameter and workplace.
| Fifth Step: Long-term validation (for the period evaluated single results) The long-term validation is to be performed individually for each parameter! |
Is the mean value of the difference greater than the formula value, may no results be issued, until the cause is cleared and removed. The process must be documented. The fields cause, corrective action, eliminated and person (correction) need to be completed. Is this not the case, "information messages" remember on it.
| In the upper half of the window you see per line the results of the long-term validation of one parameter or a workplace. Below there is a list of the single duplicate determinations. The associated semen analysis can be viewed by "double-click" or "right-click". |
| <a href="/index.php?title=MedITEX_IVF_manual">Back to the MedITEX IVF menu </a> | <a href="#top">Back to top</a> |