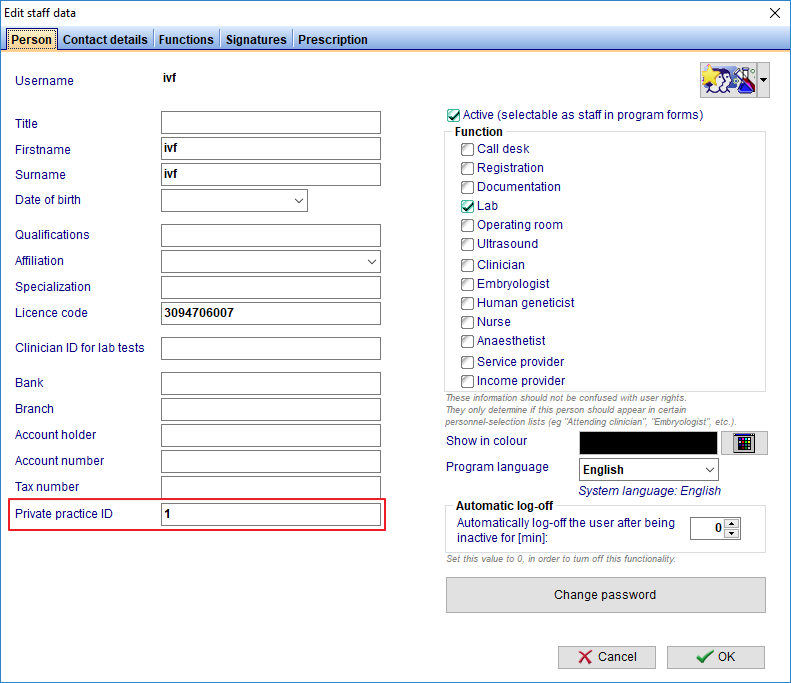
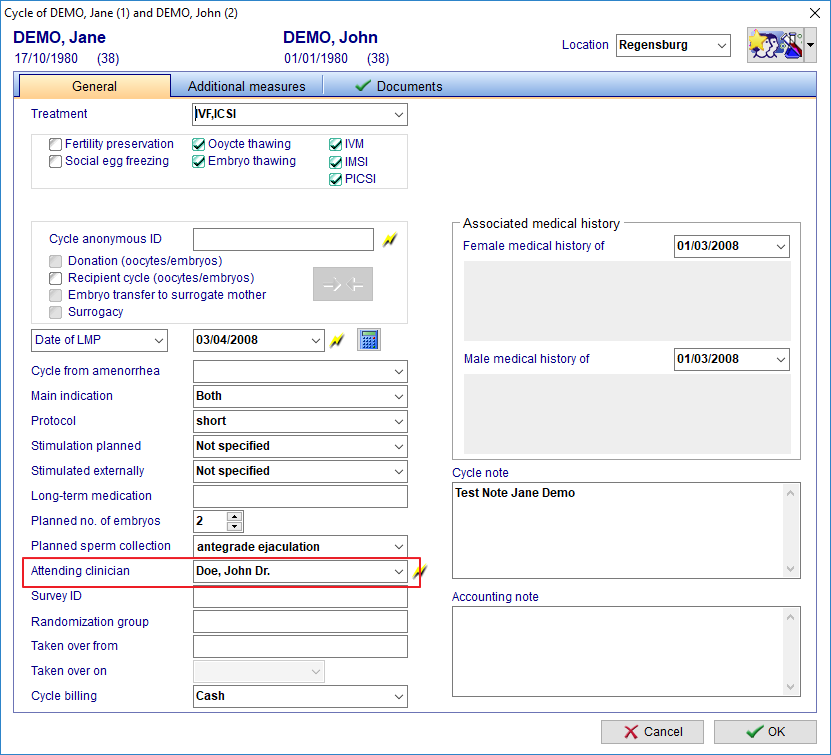
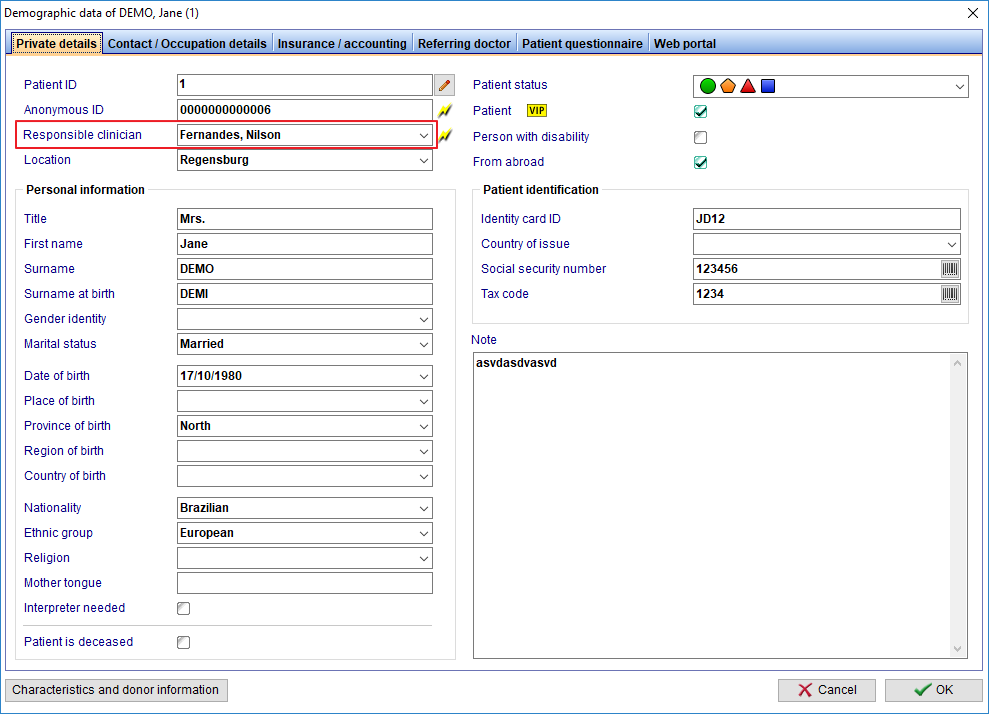
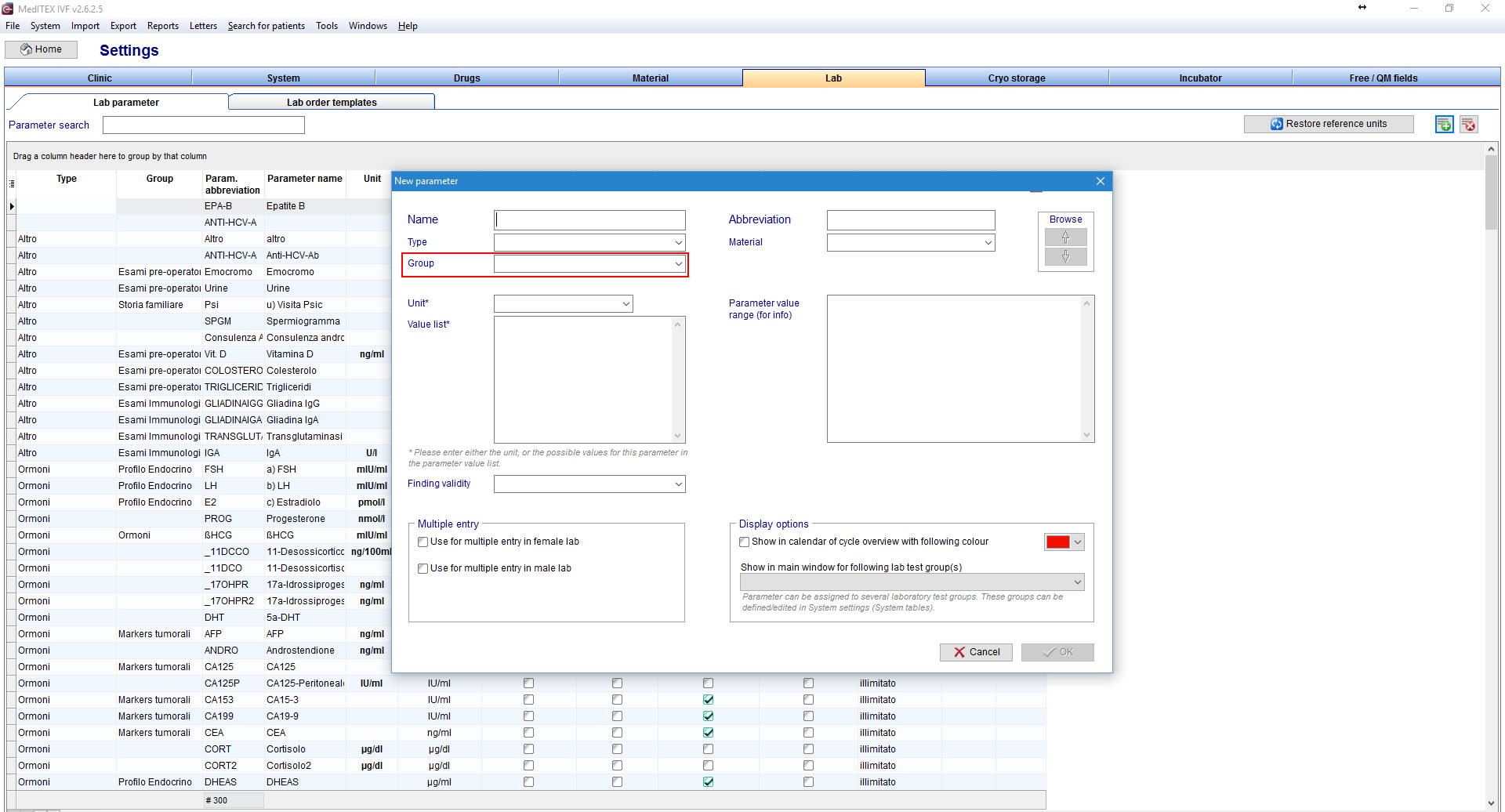
FIVNAT Export
From MedITEX - Wiki
| (22 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | < | + | <p>FIVNAT</p> |
| − | <p>MedITEX allows you to exclude patients from the FIVNAT export, by selecting the corresponding option in | + | <p>MedITEX allows you to exclude patients from the FIVNAT export, by selecting the corresponding option in <strong>Demographics -> Edit person</strong> tab.</p> |
| − | <p>Cycles included in the export are: IVF, ICSI, ICF/ICSI, Thaw cycle and Only reception.</p> | + | <p>You can also exclude a single cycle of a patient from the FIVNAT export, by selecting the corresponding option in the <strong>cycle details</strong> window:</p> |
| + | <table border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td><img title="FIVNAT cycle exclusion" src="/images/only_internal_cycle.png" alt="FIVNAT cycle exclusion" width="750" height="681" /><br /></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p><br />Cycles included in the export are: IVF, ICSI, ICF/ICSI, Thaw cycle and Only reception.</p> | ||
<p>Cycles excluded from the export are: all cycles from patients that have FIVNAT exclusion in Demographics, cycles with Reception of Ooc/Emb checkbox defined in the Cycle overview.</p> | <p>Cycles excluded from the export are: all cycles from patients that have FIVNAT exclusion in Demographics, cycles with Reception of Ooc/Emb checkbox defined in the Cycle overview.</p> | ||
| + | <p> </p> | ||
| + | <h3>Center and Subcenter ID</h3> | ||
| + | <p>Center ID comes from the system settings:</p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td><img src="/images/CenterID.png" alt="" width="744" height="639" /></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p> </p> | ||
| + | <p>Subcenter ID can come from different places, depending on the type of Export.</p> | ||
| + | <p>Each user that has a subcenter ID with FIVNAT, must have the following entry in their personal settings:</p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td>[[Image:FIVNAT_ID.png|none|740px|thumb|left|link=http://wiki.meditex-software.com/images/FIVNAT_ID.png]]</td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p> </p> | ||
| + | <p> </p> | ||
| + | <p>If the export is from Fresh cycles, it will come from Attending clinician of the Cycle details, if this field is documented:</p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td>[[Image:AttendingClinician_FIVNAT.png|none|740px|thumb|left|link=http://wiki.meditex-software.com/images/AttendingClinician_FIVNAT.png]]</td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p> </p> | ||
| + | <p>If cycle <strong>Attending clinician</strong> is not documented, it will take from <strong>Responsible clinician</strong> of Demographics area:</p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td>[[Image:ResponsCliniFIVNAT.png|none|740px|thumb|left|link=http://wiki.meditex-software.com/images/ResponsCliniFIVNAT.png]]</td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p> </p> | ||
| + | <p>In case of Destruction or Import Cycles, <strong>Respondible clinician</strong> will be taken.</p> | ||
| + | <p>If no clinician is found in Cycle details of Demographics, the ID taken will come fromt he general settings:</p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td><img src="/images/SubcenterID.png" alt="" width="744" height="641" /></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <p> </p> | ||
<p> </p> | <p> </p> | ||
<h3>Demographics</h3> | <h3>Demographics</h3> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>Date of birth</strong></td> | + | <td style="text-align: center;"><strong>Date of birth</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 14: | Line 72: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><strong>Country of residence (Female)</strong></td> | + | <td style="text-align: center;"><strong>Country of residence (Female)</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 24: | Line 82: | ||
<h3>Female medical history linked to cycle</h3> | <h3>Female medical history linked to cycle</h3> | ||
<h4>Female sterility type</h4> | <h4>Female sterility type</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 44: | Line 102: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med1.png" alt="" width="679" height="292" /> | <td><img src="/images/FIVNAT_Med1.png" alt="" width="679" height="292" /> | ||
| − | <p><strong>Female Medical History --> Fertility --> Infertility type</strong></p> | + | <p style="text-align: center;"><strong>Female Medical History --> Fertility --> Infertility type</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 55: | Line 113: | ||
<p> </p> | <p> </p> | ||
<h4>Number of previous treatments</h4> | <h4>Number of previous treatments</h4> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med2.png" alt="" width="676" height="416" /> | <td><img src="/images/FIVNAT_Med2.png" alt="" width="676" height="416" /> | ||
| − | <p><strong>Female Medical History --> Basic --> Previous treatments</strong></p> | + | <p style="text-align: center;"><strong>Female Medical History --> Basic --> Previous treatments</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 66: | Line 124: | ||
<p> </p> | <p> </p> | ||
<h4>Number of births, abortions and previous ectopic pregnancies</h4> | <h4>Number of births, abortions and previous ectopic pregnancies</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
<td> </td> | <td> </td> | ||
</tr> | </tr> | ||
| Line 109: | Line 167: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med3.png" alt="" width="676" height="451" /> | <td><img src="/images/FIVNAT_Med3.png" alt="" width="676" height="451" /> | ||
| − | <p>A new window will open then</p> | + | <p style="text-align: center;">A new window will open then</p> |
<img src="/images/FIVNAT_Med4.png" alt="" width="680" height="313" /> | <img src="/images/FIVNAT_Med4.png" alt="" width="680" height="313" /> | ||
<p> </p> | <p> </p> | ||
| − | <p><strong>Female Medical History --> Basic --> Gravida/Para Section</strong></p> | + | <p style="text-align: center;"><strong>Female Medical History --> Basic --> Gravida/Para Section</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 123: | Line 181: | ||
<p> </p> | <p> </p> | ||
<h4>Sterility factors</h4> | <h4>Sterility factors</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 191: | Line 249: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med5.png" alt="" width="676" height="447" /> | <td><img src="/images/FIVNAT_Med5.png" alt="" width="676" height="447" /> | ||
| − | <p><strong>Female Medical History --> Basic --> Sterility Factors (4+1 fields)</strong></p> | + | <p style="text-align: center;"><strong>Female Medical History --> Basic --> Sterility Factors (4+1 fields)</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 202: | Line 260: | ||
<p> </p> | <p> </p> | ||
<h4>Unprotected intercourse since</h4> | <h4>Unprotected intercourse since</h4> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med6.png" alt="" width="674" height="612" /> | <td><img src="/images/FIVNAT_Med6.png" alt="" width="674" height="612" /> | ||
| − | <p><strong>Female Medical History --> Basic --> Unprotected intercourse since (year)</strong></p> | + | <p style="text-align: center;"><strong>Female Medical History --> Basic --> Unprotected intercourse since (year)</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 214: | Line 272: | ||
<h3>Male medical history linked to cycle</h3> | <h3>Male medical history linked to cycle</h3> | ||
<h4>Male sterility type</h4> | <h4>Male sterility type</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 234: | Line 292: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med7.png" alt="" width="675" height="244" /> | <td><img src="/images/FIVNAT_Med7.png" alt="" width="675" height="244" /> | ||
| − | <p><strong>Male Medical History --> General --> Infertility type</strong></p> | + | <p style="text-align: center;"><strong>Male Medical History --> General --> Infertility type</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 245: | Line 303: | ||
<p> </p> | <p> </p> | ||
<h4>Male pathology</h4> | <h4>Male pathology</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options --> Sterility factor</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options --> Sterility factor</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 280: | Line 338: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options --> Chromosome analysis</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options --> Chromosome analysis</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 288: | Line 346: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX optsions --> CFTR carrier</strong></td> | + | <td style="text-align: center;"><strong>MedITEX optsions --> CFTR carrier</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 297: | Line 355: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med8.png" alt="" width="675" height="315" /> | <td><img src="/images/FIVNAT_Med8.png" alt="" width="675" height="315" /> | ||
| − | <p><strong>Male Medical History --> Basic</strong></p> | + | <p style="text-align: center;"><strong>Male Medical History --> Basic</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 308: | Line 366: | ||
<p> </p> | <p> </p> | ||
<h4>Male semen diagnosis</h4> | <h4>Male semen diagnosis</h4> | ||
| − | <p> | + | <p>The eight FIVNAT options related to the male semen diagnosis are taken from the drop-down menu "<strong>Diagnosis</strong>" within the semen analysis window.</p> |
| − | <p> | + | <p><strong>Here it concerns the very first semen analysis (spermiogram before) in the male Lab diagnostics</strong>. </p> |
| − | <table border="0"> | + | <p> </p> |
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | + | <td style="text-align: center;"><strong>MedITEX options --> Semen analysis</strong></td> | |
| − | </td | + | |
| − | + | ||
| − | + | ||
| − | <td | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 364: | Line 409: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Med11.png" alt="" width="657" height="496" /> | <td><img src="/images/FIVNAT_Med11.png" alt="" width="657" height="496" /> | ||
| − | <p><strong>Laboratory Diag. --> New Element --> Semen Analysis</strong></p> | + | <p style="text-align: center;"><strong>Laboratory Diag. --> New Element --> Semen Analysis </strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 376: | Line 421: | ||
<h3>Fresh and thawing cycles</h3> | <h3>Fresh and thawing cycles</h3> | ||
<h4>Cycle type</h4> | <h4>Cycle type</h4> | ||
| − | <p>The FIVNAT export query distinguishes Fresh cycles from thawing cycles, if in the Culture section, thawing of oocytes, Zygotes or embryos takes place.</p> | + | <p>The FIVNAT export query distinguishes Fresh cycles from thawing cycles, if in the Culture section, thawing of oocytes, Zygotes or embryos takes place.</p> |
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh1.png" alt="" width="143" height="98" /> | <td><img src="/images/FIVNAT_Fresh1.png" alt="" width="143" height="98" /> | ||
| − | <p><strong>Therapy --> Culture</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 388: | Line 433: | ||
<p> </p> | <p> </p> | ||
<h4>Import date</h4> | <h4>Import date</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh2.png" alt="" width="629" height="200" /> | <td><img src="/images/FIVNAT_Fresh2.png" alt="" width="629" height="200" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 399: | Line 444: | ||
<p> </p> | <p> </p> | ||
<h4>Cycle indication</h4> | <h4>Cycle indication</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 455: | Line 500: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/ | + | <td><img title="FIVNAT cycle indication" src="/images/cycle_indication.png" alt="FIVNAT cycle indication" width="750" height="681" /> |
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 466: | Line 511: | ||
<p> </p> | <p> </p> | ||
<h4>Scheduled fertilization technique</h4> | <h4>Scheduled fertilization technique</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 486: | Line 531: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh4.png" alt="" width="631" height="150" /> | <td><img src="/images/FIVNAT_Fresh4.png" alt="" width="631" height="150" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 498: | Line 543: | ||
<h4>Protocol</h4> | <h4>Protocol</h4> | ||
<p>This field should only be exported in case the cycle is Fresh. For Cryo cycles, field exported is called StimulationType (column EU).</p> | <p>This field should only be exported in case the cycle is Fresh. For Cryo cycles, field exported is called StimulationType (column EU).</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 522: | Line 567: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" /> | <td><img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 535: | Line 580: | ||
<p>The FIVNAT export Query select the start date of the first stimulation drug inserted in Cycle overview.</p> | <p>The FIVNAT export Query select the start date of the first stimulation drug inserted in Cycle overview.</p> | ||
<p>If no stimulation medication is applied for this cycle, the cycle start date in the Cycle details is taken.</p> | <p>If no stimulation medication is applied for this cycle, the cycle start date in the Cycle details is taken.</p> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh6.png" alt="" width="698" height="296" /> | <td><img src="/images/FIVNAT_Fresh6.png" alt="" width="698" height="296" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 546: | Line 591: | ||
<p> </p> | <p> </p> | ||
<h4>Drugs used in the treatment or after transfer</h4> | <h4>Drugs used in the treatment or after transfer</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 594: | Line 639: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh7.png" alt="" width="712" height="385" /> | <td><img src="/images/FIVNAT_Fresh7.png" alt="" width="712" height="385" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Overview</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p><span id="result_box" lang="en">[[Image:FIVNAT_Fresh8.png|none|740px|thumb|left|link=http://wiki.meditex-software.com/images/FIVNAT_Fresh8.png| ]]</span></p> | + | <p style="text-align: center;"><span id="result_box" lang="en"><span title="Sollte der Ordner „Updates" nicht vorhanden sein, einfach erstellen. "><span lang="en"><span class="hps"><span id="result_box" lang="en"><span><span id="result_box" lang="en"><span title="Sollte der Ordner „Updates" nicht vorhanden sein, einfach erstellen. "><span id="result_box" lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span id="result_box" lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span id="result_box" lang="en"><span title="Ob die Datei geschlossen werden darf sehen sie anhand des Eintrags der letzten Spalte. "><span id="result_box" lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span id="result_box" lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. ">[[Image:FIVNAT_Fresh8.png|none|740px|thumb|left|link=http://wiki.meditex-software.com/images/FIVNAT_Fresh8.png| ]]</span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></p> |
| − | <p><strong><span lang="en">Groups of drugs can be assigned in System --> Configuration/Administration --> Drugs</span></strong></p> | + | <p style="text-align: center;"><strong><span lang="en"><span title="Sollte der Ordner „Updates" nicht vorhanden sein, einfach erstellen. "><span lang="en"><span class="hps"><span lang="en"><span lang="en"><span title="Sollte der Ordner „Updates" nicht vorhanden sein, einfach erstellen. "><span lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span lang="en"><span title="Ob die Datei geschlossen werden darf sehen sie anhand des Eintrags der letzten Spalte. "><span lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. "><span lang="en"><span title="Nutzen sie den Refresh Button um die Ansicht zu aktualisieren. ">Groups of drugs can be assigned in System --> Configuration/Administration --> Drugs</span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></span></strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 612: | Line 657: | ||
<p> </p> | <p> </p> | ||
<h4>Failure of the stimulation and reason</h4> | <h4>Failure of the stimulation and reason</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 628: | Line 673: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh9.png" alt="" width="662" height="254" /> | <td><img src="/images/FIVNAT_Fresh9.png" alt="" width="662" height="254" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Overview</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 639: | Line 684: | ||
<p> </p> | <p> </p> | ||
<h4>Follicular aspiration date and n° of collected oocytes</h4> | <h4>Follicular aspiration date and n° of collected oocytes</h4> | ||
| − | <p>The FIVNAT export Query extracts for fresh cycles the date of Follicular Aspiration and the number of oocytes collected, while for thawing cycles extracts the date of the original follicular aspiration.</p> | + | <p>The FIVNAT export Query extracts for fresh cycles the date of Follicular Aspiration and the number of oocytes collected, while for thawing cycles extracts the date of the original follicular aspiration.</p> |
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh10.png" alt="" width="275" height="449" /> | <td><img src="/images/FIVNAT_Fresh10.png" alt="" width="275" height="449" /> | ||
| − | <p><strong>Therapy</strong><strong> | + | <p style="text-align: center;"><strong>Therapy</strong><strong> --> Aspiration</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 651: | Line 696: | ||
<p> </p> | <p> </p> | ||
<h4>Fertilization technique</h4> | <h4>Fertilization technique</h4> | ||
| − | <p>The FIVNAT export Query extracts for fresh cycles and thawing cycles where only oocytes are thawed, the technique used in culture, while thawing cycles where PN and embryos are thawed, checks what type of cells were thawed.</p> | + | <p>The FIVNAT export Query extracts for fresh cycles and thawing cycles where only oocytes are thawed, the technique used in culture, while thawing cycles where PN and embryos are thawed, checks what type of cells were thawed.</p> |
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 676: | Line 721: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh11.png" alt="" width="646" height="237" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh11.png" alt="" width="646" height="237" /> |
<p> </p> | <p> </p> | ||
| − | <img src="/images/FIVNAT_Fresh12.png" alt="" width="343" height="346" /> | + | <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh12.png" alt="" width="343" height="346" /> |
| − | <p><strong>Therapy --> Culture --> Graphical representation --> Cycle Details</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Graphical representation --> Cycle Details</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh13.png" alt="" width="457" height="455" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh13.png" alt="" width="457" height="455" /> |
| − | <p><strong> </strong>For thawing cycles, the fertilization technique is extracted from the material type thawed.</p> | + | <p style="text-align: center;"><strong> </strong>For thawing cycles, the fertilization technique is extracted from the material type thawed.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 694: | Line 739: | ||
<p> </p> | <p> </p> | ||
<h4>Origin of sperm</h4> | <h4>Origin of sperm</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options --> First 2 semen analysis linked to the cycle</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options --> First 2 semen analysis linked to the cycle</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 726: | Line 771: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh14.png" alt="" width="400" height="267" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh14.png" alt="" width="400" height="267" /> |
| − | <p><strong>Therapy --> Culture --> Treatments and treatment semen analyses (Partner/Donor, Fresh/Cryo)</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Treatments and treatment semen analyses (Partner/Donor, Fresh/Cryo)</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh15.png" alt="" width="493" height="598" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh15.png" alt="" width="493" height="598" /> |
| − | <p><strong>Laboratory Diag. --> New element --> Semen analysis</strong></p> | + | <p style="text-align: center;"><strong>Laboratory Diag. --> New element --> Semen analysis</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 741: | Line 786: | ||
</table> | </table> | ||
<h4>N° of injected oocytes</h4> | <h4>N° of injected oocytes</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh16.png" alt="" width="389" height="175" /> | <td><img src="/images/FIVNAT_Fresh16.png" alt="" width="389" height="175" /> | ||
| − | <p><strong>Therapy --> Culture --> Graphical representation</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Graphical representation</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 794: | Line 839: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh17.png" alt="" width="670" height="382" /> | <td><img src="/images/FIVNAT_Fresh17.png" alt="" width="670" height="382" /> | ||
| − | <p><strong>Therapy --> Culture --> Graphical representation</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Graphical representation</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 805: | Line 850: | ||
<p> </p> | <p> </p> | ||
<h4>N° of destructed cells</h4> | <h4>N° of destructed cells</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 825: | Line 870: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh18.png" alt="" width="273" height="258" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh18.png" alt="" width="273" height="258" /> |
| − | <p><strong>Therapy --> Culture --> Graphical Representation</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Graphical Representation</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 836: | Line 881: | ||
<p> </p> | <p> </p> | ||
<h4>Reason for embryo destruction</h4> | <h4>Reason for embryo destruction</h4> | ||
| − | <table border="0"> | + | <p>The reason for embryo destruction <strong>within the graphical representation in the colture tabsheet</strong>, has to be entered in the corresponding drop-down menu:</p> |
| + | <p> </p> | ||
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><strong>FIVNAT options</strong></td> | <td><strong>FIVNAT options</strong></td> | ||
| − | <td><strong>MedITEX options --> | + | <td><strong>MedITEX options --> Drop-Down Menu<br /></strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 856: | Line 903: | ||
<tr> | <tr> | ||
<td>Other Reasons</td> | <td>Other Reasons</td> | ||
| − | <td>Other reasons, or if I have embryos destroyed but empty | + | <td>Other reasons, or if I have embryos destroyed but empty field</td> |
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Genetic disorder</td> | ||
| + | <td>Genetic disorder</td> | ||
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/ | + | <td><img title="FIVNAT reason destruction" src="/images/reason_destruction.png" alt="FIVNAT reason destruction" width="478" height="268" /></td> |
| − | + | ||
| − | </td> | + | |
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| + | <p> </p> | ||
<p> </p> | <p> </p> | ||
<h4>Cryo cells per Cryo Technique</h4> | <h4>Cryo cells per Cryo Technique</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 889: | Line 939: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh20.png" alt="" width="656" height="551" /> | <td><img src="/images/FIVNAT_Fresh20.png" alt="" width="656" height="551" /> | ||
| − | <p><strong>Therapy --> Culture --> Right click on the cell to cryopreserve</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Right click on the cell to cryopreserve</strong></p> |
| − | <p>or</p> | + | <p style="text-align: center;">or</p> |
| − | <p><strong>Overview --> blue Square --> + button</strong></p> | + | <p style="text-align: center;"><strong>Overview --> blue Square --> + button</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 902: | Line 952: | ||
<p> </p> | <p> </p> | ||
<h4>Embryo for research reason</h4> | <h4>Embryo for research reason</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 922: | Line 972: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh21.png" alt="" width="614" height="454" /> | <td><img src="/images/FIVNAT_Fresh21.png" alt="" width="614" height="454" /> | ||
| − | <p><strong>Cryopreservation</strong></p> | + | <p style="text-align: center;"><strong>Cryopreservation</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 933: | Line 983: | ||
<p> </p> | <p> </p> | ||
<h4>Transfer date</h4> | <h4>Transfer date</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh22.png" alt="" width="668" height="250" /> | <td><img src="/images/FIVNAT_Fresh22.png" alt="" width="668" height="250" /> | ||
| − | <p><strong>Therapy --> Transfer</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Transfer</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 944: | Line 994: | ||
<p> </p> | <p> </p> | ||
<h4>Reason for cancelling transfer</h4> | <h4>Reason for cancelling transfer</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 972: | Line 1,022: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh23.png" alt="" width="672" height="183" /> | <td><img src="/images/FIVNAT_Fresh23.png" alt="" width="672" height="183" /> | ||
| − | <p><strong>Therapy --> Transfer</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Transfer</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 983: | Line 1,033: | ||
<p> </p> | <p> </p> | ||
<h4>Transferred Cells</h4> | <h4>Transferred Cells</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh24.png" alt="" width="622" height="525" /> | <td><img src="/images/FIVNAT_Fresh24.png" alt="" width="622" height="525" /> | ||
| − | <p><strong>Therapy --> Culture --> Right click on the cell to transfer</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Right click on the cell to transfer</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 995: | Line 1,045: | ||
<h4>Transfer complication</h4> | <h4>Transfer complication</h4> | ||
<p>In FIVNAT, transfer complications are separated into several fields.</p> | <p>In FIVNAT, transfer complications are separated into several fields.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,043: | Line 1,093: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh25.png" alt="" width="700" height="469" /> | <td><img src="/images/FIVNAT_Fresh25.png" alt="" width="700" height="469" /> | ||
| − | <p><strong>Therapy --> Transfer</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Transfer</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,054: | Line 1,104: | ||
<p> </p> | <p> </p> | ||
<h4>Cycle result</h4> | <h4>Cycle result</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,094: | Line 1,144: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 1,103: | Line 1,153: | ||
<p> </p> | <p> </p> | ||
<h4>Thawed cells</h4> | <h4>Thawed cells</h4> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh26.png" alt="" width="500" height="280" /> | <td><img src="/images/FIVNAT_Fresh26.png" alt="" width="500" height="280" /> | ||
| − | <p><strong>Therapy --> Culture --> Thawing</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Thawing</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,114: | Line 1,164: | ||
<p> </p> | <p> </p> | ||
<h4>UM date</h4> | <h4>UM date</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh28.png" alt="" width="630" height="182" /> | <td><img src="/images/FIVNAT_Fresh28.png" alt="" width="630" height="182" /> | ||
| − | <p><strong>Therapy --> Cycle details</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Cycle details </strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,126: | Line 1,176: | ||
<h4>Stimulation type</h4> | <h4>Stimulation type</h4> | ||
<p>This option is only exported in Cryo cycles. For Fresh cycles, field exported is called Protocol (column CB).</p> | <p>This option is only exported in Cryo cycles. For Fresh cycles, field exported is called Protocol (column CB).</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,146: | Line 1,196: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" /> | <td><img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" /> | ||
| − | <p><strong>Therapy --> Cycle Details<br /></strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Cycle Details<br /></strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,157: | Line 1,207: | ||
<p> </p> | <p> </p> | ||
<h4>Cryo cell from another center</h4> | <h4>Cryo cell from another center</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,173: | Line 1,223: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh31.png" alt="" width="650" height="471" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh31.png" alt="" width="650" height="471" /> |
| − | <p><strong>Cryopreservation</strong></p> | + | <p style="text-align: center;"><strong>Cryopreservation </strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/FIVNAT_Fresh32.png" alt="" width="550" height="582" /> | + | <td><img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh32.png" alt="" width="550" height="582" /> |
| − | <p><strong>Thawing</strong></p> | + | <p style="text-align: center;"><strong>Thawing</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,189: | Line 1,239: | ||
<p> </p> | <p> </p> | ||
<h4>Thawing date</h4> | <h4>Thawing date</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh33.png" alt="" width="550" height="241" /> | <td><img src="/images/FIVNAT_Fresh33.png" alt="" width="550" height="241" /> | ||
| − | <p><strong>Therapy --> Culture --> Thawing</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Thawing</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,200: | Line 1,250: | ||
<p> </p> | <p> </p> | ||
<h4>Cryo cells in thawing cycles</h4> | <h4>Cryo cells in thawing cycles</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh34.png" alt="" width="700" height="321" /> | <td><img src="/images/FIVNAT_Fresh34.png" alt="" width="700" height="321" /> | ||
| − | <p><strong>Therapy --> Culture --> Cryopreservation</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Culture --> Cryopreservation</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,210: | Line 1,260: | ||
</table> | </table> | ||
<p> </p> | <p> </p> | ||
| − | <h4>Reason for new cryopreservation</h4> | + | <h4>Reason for cryopreservation / new cryopreservation</h4> |
| − | <table border="0"> | + | <p>The reason for embryo cryopreservation / new cryopreservation within the graphical representation in the colture tabsheet has to be entered in the corresponding drop-down menu:</p> |
| + | <table style="margin-left: auto; margin-right: auto;" border="0"> | ||
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options --> | + | <td style="text-align: center;"><strong>MedITEX options --> Drop-Down Menu</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,251: | Line 1,302: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><img src="/images/ | + | <td><img title="FIVNAT reason cryo" src="/images/reason_cryo.png" alt="FIVNAT reason cryo" width="468" height="277" /></td> |
| − | + | ||
| − | </td> | + | |
</tr> | </tr> | ||
</tbody> | </tbody> | ||
| Line 1,262: | Line 1,311: | ||
<p> </p> | <p> </p> | ||
<h4>US</h4> | <h4>US</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 1,282: | Line 1,331: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0" width="10" height="24"> | + | <table style="margin-left: auto; margin-right: auto;" border="0" width="10" height="24"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 1,293: | Line 1,342: | ||
<p>The FIVNAT Query selects:</p> | <p>The FIVNAT Query selects:</p> | ||
<ul> | <ul> | ||
| − | <li><strong>Number induced abortions:</strong> | + | <li><strong>Number induced abortions:</strong> Each cycle in which during pregnancy one of 4 embryos has induced abortion is counted in the number of induced abortions.</li> |
</ul> | </ul> | ||
<ul> | <ul> | ||
| − | <li><strong>Date:</strong> | + | <li><strong>Date:</strong> The date is the first submitted in case of abortion.</li> |
</ul> | </ul> | ||
<ul> | <ul> | ||
| − | <li><strong>Reason:</strong> | + | <li><strong>Reason:</strong> The first indication for abortion in cases of induced abortion.</li> |
</ul> | </ul> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 1,310: | Line 1,359: | ||
<p> </p> | <p> </p> | ||
<h4>Pregnancies results</h4> | <h4>Pregnancies results</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,338: | Line 1,387: | ||
<tr> | <tr> | ||
<td>Induced Abortion</td> | <td>Induced Abortion</td> | ||
| − | <td>At least one embryo with Induced abortion as Pregnancy process until 24<sup>th</sup> | + | <td>At least one embryo with Induced abortion as Pregnancy process until 24<sup>th</sup> week</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,346: | Line 1,395: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| Line 1,352: | Line 1,401: | ||
<p> </p> | <p> </p> | ||
<img src="/images/FIVNAT_Fresh39.png" alt="" width="650" height="257" /> | <img src="/images/FIVNAT_Fresh39.png" alt="" width="650" height="257" /> | ||
| − | <p><strong>Therapy --> Pregnancies, Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Pregnancies, Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,359: | Line 1,408: | ||
<p> </p> | <p> </p> | ||
<h4>Complications</h4> | <h4>Complications</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,451: | Line 1,500: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh40.png" alt="" width="650" height="130" /> | <td><img src="/images/FIVNAT_Fresh40.png" alt="" width="650" height="130" /> | ||
| − | <p><strong>Therapy --> Pregnancy</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Pregnancy</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,462: | Line 1,511: | ||
<p> </p> | <p> </p> | ||
<h4>Delivery mode</h4> | <h4>Delivery mode</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,490: | Line 1,539: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh41.png" alt="" width="406" height="403" /> | <td><img src="/images/FIVNAT_Fresh41.png" alt="" width="406" height="403" /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,502: | Line 1,551: | ||
<h4>Delivery date</h4> | <h4>Delivery date</h4> | ||
<p>The FIVNAT query selects the first not null date of birth.</p> | <p>The FIVNAT query selects the first not null date of birth.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh42.png" alt="" width="650" height="195" /> | <td><img src="/images/FIVNAT_Fresh42.png" alt="" width="650" height="195" /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,514: | Line 1,563: | ||
<h4>Babies number</h4> | <h4>Babies number</h4> | ||
<p>The FIVNAT query counts the number of babies with birth date not null.</p> | <p>The FIVNAT query counts the number of babies with birth date not null.</p> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh43.png" alt="" width="650" height="195" /><br /> | <td><img src="/images/FIVNAT_Fresh43.png" alt="" width="650" height="195" /><br /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,525: | Line 1,574: | ||
<p> </p> | <p> </p> | ||
<h4>Babies Weight</h4> | <h4>Babies Weight</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh44.png" alt="" width="415" height="403" /><br /> | <td><img src="/images/FIVNAT_Fresh44.png" alt="" width="415" height="403" /><br /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,536: | Line 1,585: | ||
<p> </p> | <p> </p> | ||
<h4>Malformations (presence and description)</h4> | <h4>Malformations (presence and description)</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh45.png" alt="" width="395" height="83" /><br /> | <td><img src="/images/FIVNAT_Fresh45.png" alt="" width="395" height="83" /><br /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,547: | Line 1,596: | ||
<p> </p> | <p> </p> | ||
<h4>Babies sex</h4> | <h4>Babies sex</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh46.png" alt="" width="450" height="439" /><br /> | <td><img src="/images/FIVNAT_Fresh46.png" alt="" width="450" height="439" /><br /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,558: | Line 1,607: | ||
<p> </p> | <p> </p> | ||
<h4>Babies Conditions</h4> | <h4>Babies Conditions</h4> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td><strong>FIVNAT options</strong></td> | + | <td style="text-align: center;"><strong>FIVNAT options</strong></td> |
| − | <td><strong>MedITEX options</strong></td> | + | <td style="text-align: center;"><strong>MedITEX options</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 1,586: | Line 1,635: | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
| − | <table border="0"> | + | <table style="margin-left: auto; margin-right: auto;" border="0"> |
<tbody> | <tbody> | ||
<tr> | <tr> | ||
<td><img src="/images/FIVNAT_Fresh51.png" alt="" width="450" height="729" /><br /> | <td><img src="/images/FIVNAT_Fresh51.png" alt="" width="450" height="729" /><br /> | ||
| − | <p><strong>Therapy --> Birth</strong></p> | + | <p style="text-align: center;"><strong>Therapy --> Birth</strong></p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 1,596: | Line 1,645: | ||
</table> | </table> | ||
<p> </p> | <p> </p> | ||
| − | < | + | <h3>Social egg freezing cycles</h3> |
<p> </p> | <p> </p> | ||
| + | <p>According to the new FIVNAT specification the Social Egg Freezing Cycles have to be documented with following rules:</p> | ||
| + | <p> </p> | ||
| + | <ul> | ||
| + | <li>The partner's birthdate must in all social freezing cycles imperatively be <strong>01.01.1900</strong></li> | ||
| + | <li>The type of man infertility must be <strong>unknown</strong></li> | ||
| + | <li>The spermiogram before must be <strong>unknown</strong></li> | ||
| + | <li>Desire to have children since must be <strong>1900</strong></li> | ||
| + | <li>Indication for IVF/ICSI and/or accompanying disorders must be <strong>Age</strong></li> | ||
| + | <li>Transfer must be <strong>no</strong></li> | ||
| + | <li>Reason for cancellation must be <strong>Oocytes cryopreservation cycle</strong></li> | ||
| + | </ul> | ||
| + | <p> </p> | ||
| + | <p>By documenting a <strong>Social egg freezing</strong> cycle in MedITEX IVF, you have to set in the cycle details window this two option:</p> | ||
| + | <p> </p> | ||
| + | <ul> | ||
| + | <li>Treatment: <strong>only aspiration</strong></li> | ||
| + | <li>Social Egg Freezing: <strong>must be active</strong></li> | ||
| + | </ul> | ||
<table border="0"> | <table border="0"> | ||
<tbody> | <tbody> | ||
<tr> | <tr> | ||
| − | <td> | + | <td><img title="FIVNAT Social egg freezing" src="/images/social_egg_freezing.png" alt="FIVNAT Social egg freezing" width="750" height="206" /></td> |
| − | <p><a href="/index.php?title= | + | </tr> |
| + | </tbody> | ||
| + | </table> | ||
| + | <p>Therefore the cycle will be automatically sent to FIVNAT applying the above mentioned rules.</p> | ||
| + | <p> </p> | ||
| + | <p> </p> | ||
| + | <p>Click <a href="/index.php?title=FIVNAT_exported_fields">here</a> to know more about all fields exported to FIVNAT.</p> | ||
| + | <p> </p> | ||
| + | <table style="float: right;" border="0"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td style="text-align: right;"> | ||
| + | <p><span><a style="font-size: small;" href="/index.php?title=More_Exporting_Features">Back to More Exporting Features<br /></a></span></p> | ||
</td> | </td> | ||
| − | <td><a href="#top">Back to top</a | + | <td style="text-align: right;"><a href="#top">Back to top</a></td> |
</tr> | </tr> | ||
</tbody> | </tbody> | ||
</table> | </table> | ||
Latest revision as of 13:04, 22 February 2021
FIVNAT
MedITEX allows you to exclude patients from the FIVNAT export, by selecting the corresponding option in Demographics -> Edit person tab.
You can also exclude a single cycle of a patient from the FIVNAT export, by selecting the corresponding option in the cycle details window:
| <img title="FIVNAT cycle exclusion" src="/images/only_internal_cycle.png" alt="FIVNAT cycle exclusion" width="750" height="681" /> |
Cycles included in the export are: IVF, ICSI, ICF/ICSI, Thaw cycle and Only reception.
Cycles excluded from the export are: all cycles from patients that have FIVNAT exclusion in Demographics, cycles with Reception of Ooc/Emb checkbox defined in the Cycle overview.
Center and Subcenter ID
Center ID comes from the system settings:
| <img src="/images/CenterID.png" alt="" width="744" height="639" /> |
Subcenter ID can come from different places, depending on the type of Export.
Each user that has a subcenter ID with FIVNAT, must have the following entry in their personal settings:
If the export is from Fresh cycles, it will come from Attending clinician of the Cycle details, if this field is documented:
If cycle Attending clinician is not documented, it will take from Responsible clinician of Demographics area:
In case of Destruction or Import Cycles, Respondible clinician will be taken.
If no clinician is found in Cycle details of Demographics, the ID taken will come fromt he general settings:
| <img src="/images/SubcenterID.png" alt="" width="744" height="641" /> |
Demographics
| Date of birth |
| <img src="/images/FIVNAT_Demo1.png" alt="" width="689" height="265" /> |
| Country of residence (Female) |
| <img src="/images/FIVNAT_Demo2.png" alt="" width="689" height="498" /> |
Female medical history linked to cycle
Female sterility type
| FIVNAT options | MedITEX options |
| Primary | Primary |
| Secondary | Secondary |
| Unknown | If this field is left blank or if any other option is chosen |
| <img src="/images/FIVNAT_Med1.png" alt="" width="679" height="292" />
Female Medical History --> Fertility --> Infertility type |
Number of previous treatments
| <img src="/images/FIVNAT_Med2.png" alt="" width="676" height="416" />
Female Medical History --> Basic --> Previous treatments |
Number of births, abortions and previous ectopic pregnancies
| FIVNAT options | MedITEX options | |
| N° Births | N° Para |
|
| N° Abortions | N° Abort |
|
| N° Ectopic pregnancies | N° Ectopic |
|
| <img src="/images/FIVNAT_Med3.png" alt="" width="676" height="451" />
A new window will open then <img src="/images/FIVNAT_Med4.png" alt="" width="680" height="313" />
Female Medical History --> Basic --> Gravida/Para Section |
Sterility factors
| FIVNAT options | MedITEX options |
| None | None |
| Endometriosis (I/II) | Endometriosis (I/II) |
| Endometriosis (III/IV) | Endometriosis (III/IV) |
| Tube Pathology | Tube Pathology |
| Womb Fibrosis | Womb Fibrosis |
| Malformation Of The Womb | Malformation Of The Womb |
| Chronic Anovulation (PCOS) | Chronic Anovulation (PCOS) |
| Hypogonadotropic Ovarian Insufficiency (WHO III) | Hypogonadotropic Ovarian Insufficiency (WHO III) |
| Hypogonadotropic Ovarian Insufficiency (WHO I) | Hypogonadotropic Ovarian Insufficiency (WHO I) |
| Malignant Change Of The Female Genital Tract | Malignant Change Of The Female Genital Tract |
| Reappearing Miscarriage | Reappearing Miscarriage |
| Thyroid Disease | Thyroid Disease |
| DiabetesMellitus I/II | DiabetesMellitus I/II |
| Breast Cancer | Breast Cancer |
| Other | If any other option is selected in the drop-down menu of the four fields and whether the free text field is not empty |
| <img src="/images/FIVNAT_Med5.png" alt="" width="676" height="447" />
Female Medical History --> Basic --> Sterility Factors (4+1 fields) |
Unprotected intercourse since
| <img src="/images/FIVNAT_Med6.png" alt="" width="674" height="612" />
Female Medical History --> Basic --> Unprotected intercourse since (year) |
Male medical history linked to cycle
Male sterility type
| FIVNAT options | MedITEX options |
| Primary | Primary |
| Secondary | Secondary |
| Unknown | If this field is left blank or if any other option is chosen |
| <img src="/images/FIVNAT_Med7.png" alt="" width="675" height="244" />
Male Medical History --> General --> Infertility type |
Male pathology
| FIVNAT options | MedITEX options --> Sterility factor |
| None | None |
| Varicocele | Varicocele |
| Status after varicocele | Status after varicocele |
| Klinefelter Syndrome | Klinefelter Syndrome |
| Paraplegia o Tetraplegia | Paraplegia o Tetraplegia |
| Status after Testicle Neoplasia | Status after Testicle Neoplasia |
| Other | If any other option is selected in the drop-down menu of the four fields and whether the free text field is not empty |
| FIVNAT options | MedITEX options --> Chromosome analysis |
| Chromosome Anomaly Numerical Or Structured | Positive results |
| FIVNAT options | MedITEX optsions --> CFTR carrier |
| Mutation Of The CFTR Genes | Yes (carrier or ill) |
| <img src="/images/FIVNAT_Med8.png" alt="" width="675" height="315" />
Male Medical History --> Basic |
Male semen diagnosis
The eight FIVNAT options related to the male semen diagnosis are taken from the drop-down menu "Diagnosis" within the semen analysis window.
Here it concerns the very first semen analysis (spermiogram before) in the male Lab diagnostics.
| FIVNAT options | MedITEX options --> Semen analysis |
| Normozoospermia | Normozoospermia |
| Oligospermia | Oligospermia |
| Asthenozoospermia | Asthenozoospermia |
| Teratozoospermia | Teratozoospermia |
| Azoospermia | Azoospermia |
| Cryptozoospermia | Cryptozoospermia |
| Other | Other |
| Unknown | If field is blank for all four semen analysis |
| <img src="/images/FIVNAT_Med11.png" alt="" width="657" height="496" />
Laboratory Diag. --> New Element --> Semen Analysis |
Fresh and thawing cycles
Cycle type
The FIVNAT export query distinguishes Fresh cycles from thawing cycles, if in the Culture section, thawing of oocytes, Zygotes or embryos takes place.
| <img src="/images/FIVNAT_Fresh1.png" alt="" width="143" height="98" />
Therapy --> Culture |
Import date
| <img src="/images/FIVNAT_Fresh2.png" alt="" width="629" height="200" />
Therapy --> Cycle Details |
Cycle indication
| FIVNAT options | MedITEX options |
| Tubal | Tubal |
| Anovulation/Dysovulation/PCO Syndrome | Anovulation/Dysovulation/PCO Syndrome |
| Endometriosis | Endometriosis |
| Male Infertility | Male Infertility |
| Male Immunological Infertility | Male Immunological Infertility |
| Donor Sperm | Donor Sperm |
| Unexplained Infertility | Unexplained Infertility |
| Genetical Cause Of The Infertility | Genetical Cause Of The Infertility |
| PGD | PGD |
| Hepatitis | Hepatitis |
| HIV | HIV |
| Other | If you select any other option or field is left blank |
| <img title="FIVNAT cycle indication" src="/images/cycle_indication.png" alt="FIVNAT cycle indication" width="750" height="681" />
Therapy --> Cycle Details |
Scheduled fertilization technique
| FIVNAT options | MedITEX options |
| IVF | IVF |
| ICSI | ICSI |
| MIXED | IVF/ICSI |
| <img src="/images/FIVNAT_Fresh4.png" alt="" width="631" height="150" />
Therapy --> Cycle Details |
Protocol
This field should only be exported in case the cycle is Fresh. For Cryo cycles, field exported is called StimulationType (column EU).
| FIVNAT options | MedITEX options |
| Short | Short |
| Long | Long |
| Natural | Natural cycle |
| Other stimulation | Option Other or any remaining option |
| <img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" />
Therapy --> Cycle Details |
Stimulation start date
The FIVNAT export Query select the start date of the first stimulation drug inserted in Cycle overview.
If no stimulation medication is applied for this cycle, the cycle start date in the Cycle details is taken.
| <img src="/images/FIVNAT_Fresh6.png" alt="" width="698" height="296" />
Therapy --> Cycle Details |
Drugs used in the treatment or after transfer
| FIVNAT options | MedITEX options |
| GnRH Agonist | GnRH Agonist |
| GnRH Antagonist | GnRH Antagonist |
| Clomifen | Clomifen |
| hmg | hMG |
| hcg | rec hCG or u hCG |
| uFSH | uFSH |
| rFSH | recFSH |
| rLH | recLH |
| Aromatase Hemmer | --- |
| Other | If other drugs are present in therapy and do not belong to the preceding groups |
| <img src="/images/FIVNAT_Fresh7.png" alt="" width="712" height="385" />
Therapy --> Overview |
|
Groups of drugs can be assigned in System --> Configuration/Administration --> Drugs |
Failure of the stimulation and reason
| FIVNAT options | MedITEX options |
| No Reaction | No follicular growth |
| Other | If you choose any other option |
| <img src="/images/FIVNAT_Fresh9.png" alt="" width="662" height="254" />
Therapy --> Overview |
Follicular aspiration date and n° of collected oocytes
The FIVNAT export Query extracts for fresh cycles the date of Follicular Aspiration and the number of oocytes collected, while for thawing cycles extracts the date of the original follicular aspiration.
| <img src="/images/FIVNAT_Fresh10.png" alt="" width="275" height="449" />
Therapy --> Aspiration |
Fertilization technique
The FIVNAT export Query extracts for fresh cycles and thawing cycles where only oocytes are thawed, the technique used in culture, while thawing cycles where PN and embryos are thawed, checks what type of cells were thawed.
| FIVNAT options | MedITEX options |
| IVF | FIVET |
| ICSI | ICSI |
| Mixed | FIVET/ICSI |
| ICSI After IVM | ICSI and IVM |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh11.png" alt="" width="646" height="237" />
<img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh12.png" alt="" width="343" height="346" /> Therapy --> Culture --> Graphical representation --> Cycle Details |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh13.png" alt="" width="457" height="455" />
For thawing cycles, the fertilization technique is extracted from the material type thawed. |
Origin of sperm
| FIVNAT options | MedITEX options --> First 2 semen analysis linked to the cycle |
| Partner | Partner |
| Donor sperm (priority if partner and donor sperm are selected) | Donor |
| Recent (priority if recent and cryo selected) | Fresh+Cryo Semen Analysis |
| Cryo | Cryo Semen Analysis |
| Ejaculate | Ejaculate |
| Epididymis | MESA fresh/cryo, PESA fresh/cryo |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh14.png" alt="" width="400" height="267" />
Therapy --> Culture --> Treatments and treatment semen analyses (Partner/Donor, Fresh/Cryo) |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh15.png" alt="" width="493" height="598" />
Laboratory Diag. --> New element --> Semen analysis |
N° of injected oocytes
| <img src="/images/FIVNAT_Fresh16.png" alt="" width="389" height="175" />
Therapy --> Culture --> Graphical representation |
| FIVNAT options | MedITEX options |
| MI | MI |
| MII | MII |
| GV | GV |
| Non Fert (day 1) | GV, MI, MII, degen.,0PN, >3PN (Day 1) |
| 1PN (day 1) | 1PN (day 1) |
| 2PN (day 1) | 2PN (day 1) |
| 3PN (day 1) | 3PN (day 1) |
| Zygotes left in culture | Zygotes selected for embryo culture |
| Embryo (day 2) | Embryo (day 2) |
| <img src="/images/FIVNAT_Fresh17.png" alt="" width="670" height="382" />
Therapy --> Culture --> Graphical representation |
N° of destructed cells
| FIVNAT options | MedITEX options |
| Oocytes Destroyed | Degenerated and Discarded day 0 |
| Zygotes Destroyed | 2pn Stopped +1pn+3pn+ 2PN Discarded day 1 |
| Embyo Destroyed | Discarded and Stopped Embryo |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh18.png" alt="" width="273" height="258" />
Therapy --> Culture --> Graphical Representation |
Reason for embryo destruction
The reason for embryo destruction within the graphical representation in the colture tabsheet, has to be entered in the corresponding drop-down menu:
| FIVNAT options | MedITEX options --> Drop-Down Menu |
| Evolution stop | Evolution stop |
| Very low development potential | Very low development potential |
| Decision by the pair | Decision by the pair |
| Other Reasons | Other reasons, or if I have embryos destroyed but empty field |
| Genetic disorder | Genetic disorder |
| <img title="FIVNAT reason destruction" src="/images/reason_destruction.png" alt="FIVNAT reason destruction" width="478" height="268" /> |
Cryo cells per Cryo Technique
| FIVNAT options | MedITEX options |
|
Oocytes/Zygotes/Embryo Verification Slow freezing |
Ovociti/Zigoti/Embrioni Verification Slow freezing |
| <img src="/images/FIVNAT_Fresh20.png" alt="" width="656" height="551" />
Therapy --> Culture --> Right click on the cell to cryopreserve or Overview --> blue Square --> + button |
Embryo for research reason
| FIVNAT options | MedITEX options |
| Evolution stop | Evolution stop |
| Very low development potential | Very low development potential |
| Decision by the pair | Decision by the pair |
| <img src="/images/FIVNAT_Fresh21.png" alt="" width="614" height="454" />
Cryopreservation |
Transfer date
| <img src="/images/FIVNAT_Fresh22.png" alt="" width="668" height="250" />
Therapy --> Transfer |
Reason for cancelling transfer
| FIVNAT options | MedITEX options |
| Puncture Failure | No oocytes aspirated |
| Fertilization Failure | No fertilization |
| Cell Division Failure | PN in arrest |
| Embryos in arrest | Embryos in arrest |
| Other | If any other reason given, besides "No cancelation" and "Not specified" |
| <img src="/images/FIVNAT_Fresh23.png" alt="" width="672" height="183" />
Therapy --> Transfer |
Transferred Cells
| <img src="/images/FIVNAT_Fresh24.png" alt="" width="622" height="525" />
Therapy --> Culture --> Right click on the cell to transfer |
Transfer complication
In FIVNAT, transfer complications are separated into several fields.
| FIVNAT options | MedITEX options |
| Hospital - OHSS | OHSS |
| Hospital - removal complication | Removal complication |
| Hospital cryo -Static OHSS | Static OHSS |
| Hospital cryo -Other | Other |
| Ambulant OHSS | Ambulant OHSS |
| Peritonitis | Peritonitis |
| Hemoperitoneum | Hemoperitoneum |
| Mental sickness | Mental sickness |
| Thromboembolism | Thromboembolism |
| Adnexal torsion | Adnexitis |
| <img src="/images/FIVNAT_Fresh25.png" alt="" width="700" height="469" />
Therapy --> Transfer |
Cycle result
| FIVNAT options | MedITEX options |
| No Pregnancy | If there is transfer and No pregnancy is selected |
| Biochemical Pregnancy | If there is transfer and PG test is performed and No pregnancy is selected |
| Clinical Pregnancy | If there is transfer and Clinical pregnancy is selected |
| Stimulation Failure | If cycle was cancelled |
| Fertilization Failure | If fertilized oocytes + 2PN =0 |
| Cell Division Failure | If there are fertilized oocytes but number of Embryos is 0 |
| Aborted Transfer Ohss | If Reason for no ET in Culture is different from No cancellation and Not specified |
| Unknown | If there is transfer and Unknown is selected |
| <img src="/images/FIVNAT_cycle1.png" alt="" width="750" height="677" /> |
Thawed cells
| <img src="/images/FIVNAT_Fresh26.png" alt="" width="500" height="280" />
Therapy --> Culture --> Thawing |
UM date
| <img src="/images/FIVNAT_Fresh28.png" alt="" width="630" height="182" />
Therapy --> Cycle details |
Stimulation type
This option is only exported in Cryo cycles. For Fresh cycles, field exported is called Protocol (column CB).
| FIVNAT options | MedITEX options |
| Natural Cycle | Natural Cycle |
| Artificial Cycle Treatment Endometrium | Artificial cycle |
| Ovarial Stimulation | If Ovarial stimulation is selected or any other option besides the ones above, in case of Cryo cycle |
| <img src="/images/FIVNAT_Fresh5.png" alt="" width="630" height="257" />
Therapy --> Cycle Details |
Cryo cell from another center
| FIVNAT options | MedITEX options |
| From the Center | If the field Center of origin in cryopreservation or thaw mask is empty |
| From Another Centre | If the field Center of origin in cryopreservation or thaw mask is not empty |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh31.png" alt="" width="650" height="471" />
Cryopreservation |
| <img style="display: block; margin-left: auto; margin-right: auto;" src="/images/FIVNAT_Fresh32.png" alt="" width="550" height="582" />
Thawing |
Thawing date
| <img src="/images/FIVNAT_Fresh33.png" alt="" width="550" height="241" />
Therapy --> Culture --> Thawing |
Cryo cells in thawing cycles
| <img src="/images/FIVNAT_Fresh34.png" alt="" width="700" height="321" />
Therapy --> Culture --> Cryopreservation |
Reason for cryopreservation / new cryopreservation
The reason for embryo cryopreservation / new cryopreservation within the graphical representation in the colture tabsheet has to be entered in the corresponding drop-down menu:
| FIVNAT options | MedITEX options --> Drop-Down Menu |
| Ohss | Ohss |
| Concomitant Disease | Concomitant Disease |
| Hemorrhage | Hemorrhage |
| Avoidance Multiples | Avoidance Multiples |
| Cervical Stenosis | Cervical Stenosis |
| Accident | Accident |
| Other | Other |
| None | None |
| <img title="FIVNAT reason cryo" src="/images/reason_cryo.png" alt="FIVNAT reason cryo" width="468" height="277" /> |
US
| FIVNAT options | MedITEX options |
| Amnion | Intrauterine and extrauterine cavity greater than zero |
| Heart Action | At least one Fetal heartbeat present (BCF) |
| shcg (default) | Otherwise |
| <img src="/images/FIVNAT_Fresh36.png" alt="" width="750" height="169" /> |
Spontaneous or induced abortions
The FIVNAT Query selects:
- Number induced abortions: Each cycle in which during pregnancy one of 4 embryos has induced abortion is counted in the number of induced abortions.
- Date: The date is the first submitted in case of abortion.
- Reason: The first indication for abortion in cases of induced abortion.
| <img src="/images/FIVNAT_Fresh37.png" alt="" width="500" height="233" /> |
Pregnancies results
| FIVNAT options | MedITEX options |
| Birth | At least one birth date present |
| Miscarriage | Spontaneous abortion for all embryos |
| Miscarriage Between 3 and 6 Months | Spontaneous abortion for all embryos and abortion date - clinical pregnancy date between 3 and 6 months |
| Ectopic Pregnancy | N° ectopic cavity different than 0 and N° intrauterine cavity equal 0 |
| Ectopic Intrauterine Pregnancy | N° ectopic cavity different than 0 and N° intrauterine cavity different than 0 |
| Induced Abortion | At least one embryo with Induced abortion as Pregnancy process until 24th week |
| Unknown | Unknown in the pregnancy result field |
| <img src="/images/FIVNAT_Fresh38.png" alt="" width="650" height="608" />
<img src="/images/FIVNAT_Fresh39.png" alt="" width="650" height="257" /> Therapy --> Pregnancies, Therapy --> Birth |
Complications
| FIVNAT options | MedITEX options |
| Menstruation 1 | Menstruation 1 |
| Menstruation 2 | Menstruation 2 |
| Menstruation 3 | Menstruation 3 |
| Gestation Diabetes | Gestation Diabetes |
| Insufficiency | Insufficiency |
| Preterm Labor 2 | Preterm Labor 2 |
| Preterm Labor 3 | Preterm Labor 3 |
| Plazenta Praevia | Plazenta Praevia |
| Septicemia | Septicemia |
| Solated Hypertonia | Solated Hypertonia |
| Premature Rupture Of Membranes | Premature Rupture Of Membranes |
| Preeclampsia | Preeclampsia |
| Eclampsia | Eclampsia |
| Psychological Problems | Psychological Problems |
| Intrauterine Growth Restriction | Intrauterine Growth Restriction |
| Hospitalization 1 | Hospitalization 1 |
| Hospitalization 2 | Hospitalization 2 |
| Hospitalization 3 | Hospitalization 3 |
| Cholestasis | Cholestasis |
| Unknown | Unknown |
| Other | Other |
| <img src="/images/FIVNAT_Fresh40.png" alt="" width="650" height="130" />
Therapy --> Pregnancy |
Delivery mode
| FIVNAT options | MedITEX options |
| Spontaneously | At least one baby has Spontaneous as Delivery mode |
| Forceps | At least one baby has Forceps as Delivery mode |
| Vacuum | At least one baby has Vacuum as Delivery mode |
| Cesarean | At least one baby has Cesarean as Delivery mode |
| Unknown | Any other option |
| <img src="/images/FIVNAT_Fresh41.png" alt="" width="406" height="403" />
Therapy --> Birth |
Delivery date
The FIVNAT query selects the first not null date of birth.
| <img src="/images/FIVNAT_Fresh42.png" alt="" width="650" height="195" />
Therapy --> Birth |
Babies number
The FIVNAT query counts the number of babies with birth date not null.
| <img src="/images/FIVNAT_Fresh43.png" alt="" width="650" height="195" /> Therapy --> Birth |
Babies Weight
| <img src="/images/FIVNAT_Fresh44.png" alt="" width="415" height="403" /> Therapy --> Birth |
Malformations (presence and description)
| <img src="/images/FIVNAT_Fresh45.png" alt="" width="395" height="83" /> Therapy --> Birth |
Babies sex
| <img src="/images/FIVNAT_Fresh46.png" alt="" width="450" height="439" /> Therapy --> Birth |
Babies Conditions
| FIVNAT options | MedITEX options |
| Healthy | Condition in Birth section: Normale |
| Intrau. Death | Condition in Birth section: Morte fetale intrauterina |
| Neonat Death Day 1 Till 6 | The difference between postpartum death date and birth date between 1-6 days |
| Neonat Death Day 7 Till 28 | The difference between postpartum death date and birth date between 7-28 days |
| Living With Complications | Congenital malformations or chromosomal abnormalities |
| <img src="/images/FIVNAT_Fresh51.png" alt="" width="450" height="729" /> Therapy --> Birth |
Social egg freezing cycles
According to the new FIVNAT specification the Social Egg Freezing Cycles have to be documented with following rules:
- The partner's birthdate must in all social freezing cycles imperatively be 01.01.1900
- The type of man infertility must be unknown
- The spermiogram before must be unknown
- Desire to have children since must be 1900
- Indication for IVF/ICSI and/or accompanying disorders must be Age
- Transfer must be no
- Reason for cancellation must be Oocytes cryopreservation cycle
By documenting a Social egg freezing cycle in MedITEX IVF, you have to set in the cycle details window this two option:
- Treatment: only aspiration
- Social Egg Freezing: must be active
| <img title="FIVNAT Social egg freezing" src="/images/social_egg_freezing.png" alt="FIVNAT Social egg freezing" width="750" height="206" /> |
Therefore the cycle will be automatically sent to FIVNAT applying the above mentioned rules.
Click <a href="/index.php?title=FIVNAT_exported_fields">here</a> to know more about all fields exported to FIVNAT.
|
<a style="font-size: small;" href="/index.php?title=More_Exporting_Features">Back to More Exporting Features |
<a href="#top">Back to top</a> |